Water Vapor Saturation Pressure: Data, Tables & Calculator
Online calculator, figures and tables with water saturation (vapor) pressure at temperatures ranging 0 to 370 °C (32 to 700°F) - in Imperial and SI Units.
Water tends to evaporate or vaporize by projecting molecules into the space above its surface. If the space is confined the partial pressure exerted by the molecules increases until the rate at which molecules reenter the liquid is equal to the rate at which they leave. The vapour pressure of water is the pressure at which water vapour is in thermodynamic equilibrium with its condensed state . At higher pressures water would condense . At this equilibrium condition the vapor pressure is the saturation pressure .
Online Water saturation pressure Calculator
The calculator below can be used to calculate the water saturation pressure at given temperatures.
The output pressure is given as kPa, bar, atm, psi (lb/in2) and psf (lb/ft2).
Temperatur must be within the ranges 0 - 370 °C, 32 - 700 °F, 273 - 645 K and 492 - 1160 °R
The saturation pressure of water depends on temperature as shown below:
See Water and Heavy Water for thermodynamic properties at standard condtions.
See also other properties of Water at varying temperature and pressure : Boiling points at high pressure, Boiling points at vacuum pressure, Density and specific weight, Dynamic and kinematic viscosity, Enthalpy and entropy, Heat of vaporization, Ionization Constant, pKw , of normal and heavy water, Melting points at high pressure, Prandtl number, Properties at Gas-Liquid Equilibrium Conditions, Specific gravity, Specific heat (heat capacity), Specific volume, Thermal conductivity, Thermal diffusivity and Vapour pressure at gas-liquid equilibrium.
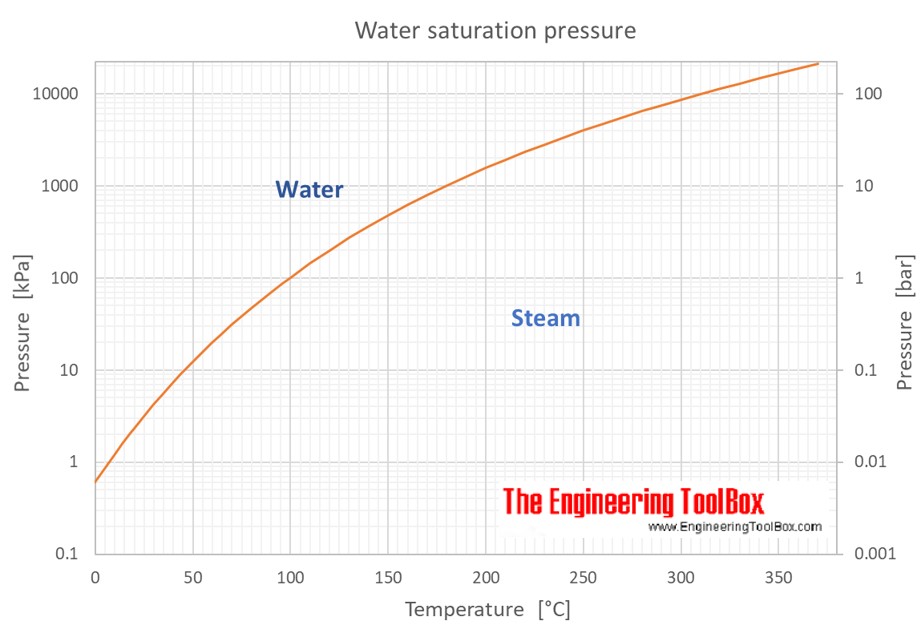
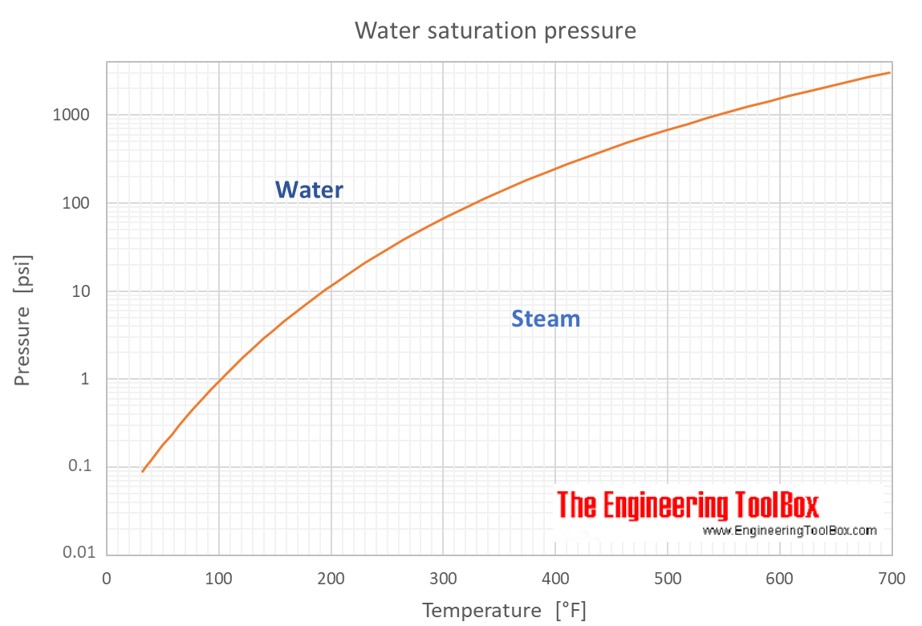
Saturation pressure at temperatures given in degree Celcius and pressure given in kiloPascals (kPa), bars, atmospheres (atm) and pounds per square inch (psi):
| Temperature | Water saturation pressure | ||
|---|---|---|---|
| (°C) | (kPa), (100*bar) | (atm) | (psi) |
| 0.01 | 0.61165 | 0.0060 | 0.088712 |
| 2 | 0.70599 | 0.0070 | 0.10240 |
| 4 | 0.81355 | 0.0080 | 0.11800 |
| 10 | 1.2282 | 0.0121 | 0.17814 |
| 14 | 1.5990 | 0.0158 | 0.23192 |
| 18 | 2.0647 | 0.0204 | 0.29946 |
| 20 | 2.3393 | 0.0231 | 0.33929 |
| 25 | 3.1699 | 0.0313 | 0.45976 |
| 30 | 4.2470 | 0.0419 | 0.61598 |
| 34 | 5.3251 | 0.0526 | 0.77234 |
| 40 | 7.3849 | 0.0729 | 1.0711 |
| 44 | 9.1124 | 0.0899 | 1.3216 |
| 50 | 12.352 | 0.122 | 1.7915 |
| 54 | 15.022 | 0.148 | 2.1788 |
| 60 | 19.946 | 0.197 | 2.8929 |
| 70 | 31.201 | 0.308 | 4.5253 |
| 80 | 47.414 | 0.468 | 6.8768 |
| 90 | 70.182 | 0.693 | 10.179 |
| 96 | 87.771 | 0.866 | 12.730 |
| 100 | 101.42 | 1.001 | 14.710 |
| 110 | 143.38 | 1.42 | 20.796 |
| 120 | 198.67 | 1.96 | 28.815 |
| 130 | 270.28 | 2.67 | 39.201 |
| 140 | 361.54 | 3.57 | 52.437 |
| 150 | 476.16 | 4.70 | 69.061 |
| 160 | 618.23 | 6.10 | 89.667 |
| 180 | 1002.8 | 9.90 | 145.44 |
| 200 | 1554.9 | 15.35 | 225.52 |
| 220 | 2319.6 | 22.89 | 336.43 |
| 240 | 3346.9 | 33.03 | 485.43 |
| 260 | 4692.3 | 46.31 | 680.56 |
| 280 | 6416.6 | 63.33 | 930.65 |
| 300 | 8587.9 | 84.76 | 1245.6 |
| 320 | 11284 | 111.4 | 1636.6 |
| 340 | 14601 | 144.1 | 2117.7 |
| 360 | 18666 | 184.2 | 2707.3 |
| 370 | 21044 | 207.7 | 3052.2 |
Saturation pressure at temperatures given in degree Fahrenheit and pressure given in pounds per square inch (psi), pounds per square foot (psf), kiloPascals (kPa) and bars:
| Temperature | Water saturation pressure | ||
|---|---|---|---|
| (°F) | (psi) | (psf) | (kPa), (100*bar) |
| 32.02 | 0.088712 | 12.775 | 0.612 |
| 34 | 0.09624 | 13.858 | 0.664 |
| 39.2 | 0.11800 | 16.991 | 0.814 |
| 40 | 0.12170 | 17.524 | 0.839 |
| 50 | 0.17814 | 25.651 | 1.228 |
| 60 | 0.25633 | 36.912 | 1.767 |
| 70 | 0.36341 | 52.330 | 2.506 |
| 80 | 0.50759 | 73.092 | 3.500 |
| 90 | 0.69915 | 100.7 | 4.821 |
| 100 | 0.95055 | 136.9 | 6.554 |
| 110 | 1.2766 | 183.8 | 8.802 |
| 120 | 1.6949 | 244.1 | 11.686 |
| 130 | 2.2258 | 320.5 | 15.347 |
| 140 | 2.8929 | 416.6 | 19.946 |
| 150 | 3.7232 | 536.1 | 25.671 |
| 160 | 4.7474 | 683.6 | 32.732 |
| 170 | 5.9999 | 864.0 | 41.368 |
| 180 | 7.5196 | 1083 | 51.846 |
| 190 | 9.3495 | 1346 | 64.462 |
| 200 | 11.537 | 1661 | 79.547 |
| 212 | 14.710 | 2118 | 101.42 |
| 220 | 17.203 | 2477 | 118.6 |
| 240 | 25.001 | 3600 | 172.4 |
| 260 | 35.263 | 5078 | 243.1 |
| 280 | 49.286 | 7097 | 339.8 |
| 300 | 67.264 | 9686 | 463.8 |
| 350 | 134.73 | 19402 | 929.0 |
| 400 | 247.01 | 35570 | 1703.1 |
| 450 | 422.32 | 60814 | 2911.8 |
| 500 | 680.56 | 98001 | 4692.3 |
| 550 | 1045.0 | 150485 | 7205.3 |
| 600 | 1542.1 | 222066 | 10632.6 |
| 625 | 1851.2 | 266570 | 12763 |
| 650 | 2207.8 | 317922 | 15222 |
| 675 | 2618.7 | 377092 | 18055 |
| 700 | 3092.0 | 445243 | 21318 |
See also Air Psychrometrics and Steam & Condensate Systems
Saturation Vapor Pressure of some other Liquids at 68 oF or 20 oC
| Liquid | Saturation Vapor Pressure | |
|---|---|---|
| (psi) | (Pa) | |
| Carbon tetrachloride,CCl4 | 1.9 | 13100 |
| Gasoline | 8.0 | 55200 |
| Mercury | 0.000025 | 0.17 |
- 1 Pa = 10-6 N/mm2 = 10-5 bar = 0.1020 kp/m2 = 1.02×10-4 m H2O = 9.869×10-6 atm = 1.45×10-4 psi (lbf/in2)



