Comprehensive Guide to Temperature Scales and Conversion
Introduction to temperature - including Celsius, Fahrenheit, Kelvin and Rankine definitions - and an online temperature converter.
Temperature (sometimes called thermodynamic temperature) is a measure of the average kinetic energy of the particles in a system. Temperature is the degree of " hotness " ( or " coldness ") - a measure of the heat intensity . The most common symbol or abbreviation for temperature is T .
When two objects of different temperatures are in contact - the warmer object becomes colder while the colder object becomes warmer. This means that heat flows from the warmer to the colder object.
Temperature Converter
Convert between °C (Celsius), °F (Fahrenheit), K (Kelvin) and °R (Rankine) with the calculator below:
| Rankine | Kelvin | Fahrenheit | Celsius |
|---|---|---|---|
| °R | K | °F | C |
| 0 | 0 | -459.67 | -273.15 |
| 100 | 55.56 | -359.67 | -217.59 |
| 180 | 100 | -279.67 | -173.15 |
| 459.67 | 255.37 | 0 | -17.78 |
| 491.67 | 273.15 | 32 | 0 |
| 559.67 | 310.93 | 100 | 37.78 |
| 671.67 | 373.15 | 212 | 100 |
Temperature Converter - Web App
Add the Temperature Converter Web App to your mobile device or desktop. The App is saved in your browser and works offline automatically after first visit.
Degree Celsius (°C) and Degree Fahrenheit (°F)
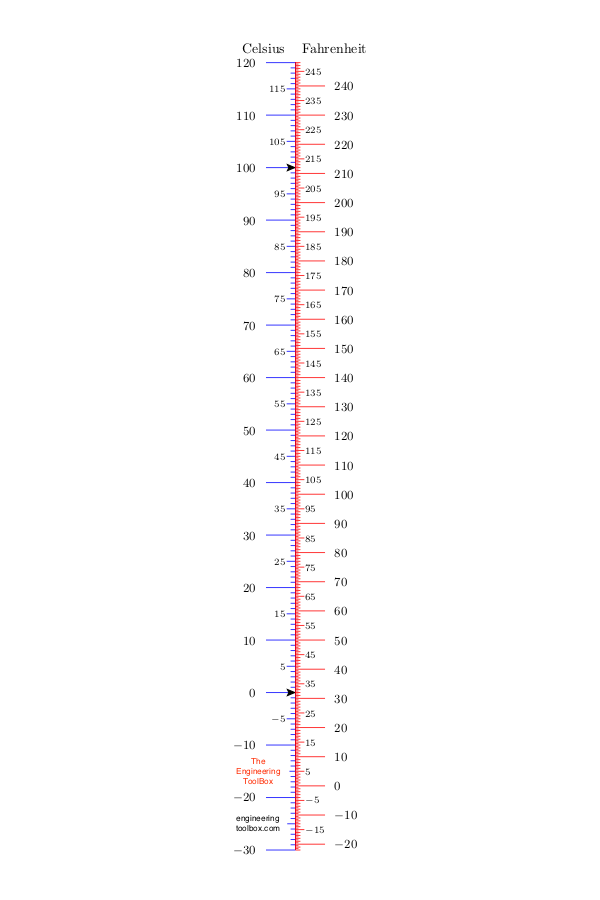
A thermometer can help us determine how cold or hot a substance is. Temperature is in most of the world measured and reported in degrees Celsius (°C). In the U.S. it is common to report temperature in degrees Fahrenheit (°F) . In the Celsius and Fahrenheit scales the temperatures where ice melts (water freezes) and water boils are used as reference points.
- In the Celsius scale the freezing point of water is defined as 0 °C and the boiling point is defined as 100 °C
- In the Fahrenheit scale the water freezes at 32 °F and boils at 212 °F
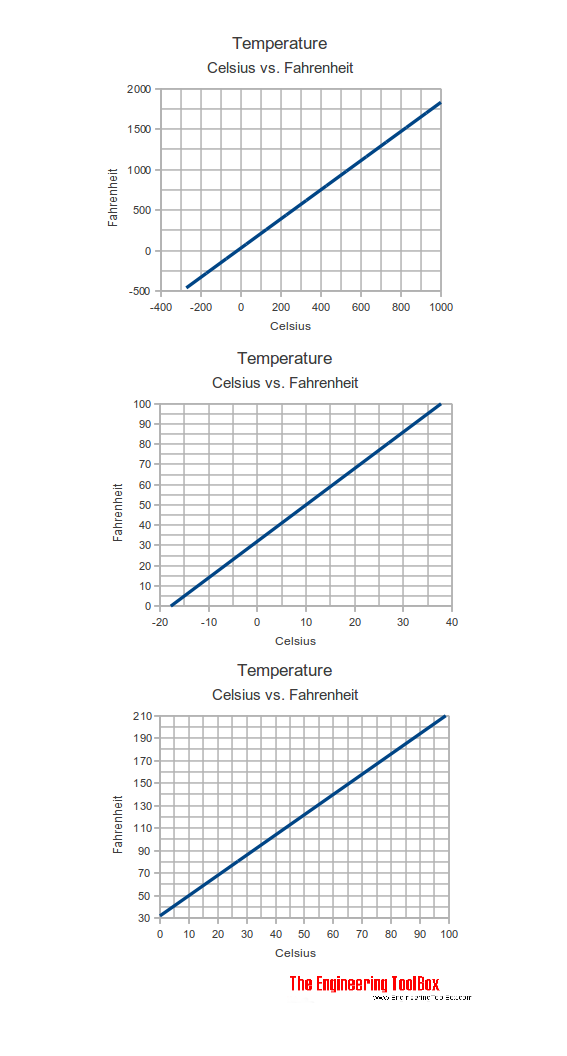
In the Celsius scale there are 100 degrees between the freezing point and the boiling point of water compared to 180 degrees in the Fahrenheit scale. This means that 1 °C = 1.8 °F (check the section about temperature differences below).
Values can be converted between the two temperature units by using the equations:
T (°F) = 1.8 T (°C) + 32 (1)
T (°C) = (T (°F) - 32) / 1.8 (2)
where
T (°C) = temperature (°C)
T (°F) = temperature (°F)
Celsius vs. Fahrenheit
| Temperature | |
|---|---|
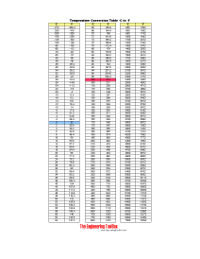
| oC | oF |
| -20 | -4 |
| -15 | 5 |
| -10 | 14 |
| -5 | 23 |
| 0 | 32 |
| 5 | 41 |
| 10 | 50 |
| 15 | 59 |
| 20 | 68 |
| 25 | 77 |
| 30 | 86 |
| 35 | 95 |
| 40 | 104 |
| 45 | 113 |
| 50 | 122 |
Example : A patient with SARS (Severe Acute Respiratory Syndrome) has a temperature of 106 °F. What is the temperature in Celsius?
T(°C) = (106 °F - 32) / 1.8
= 41.1 °C
Temperature Conversion Table - oC vs °F
Temperature Difference - or Temperature Change - degree Celsius versus degree Fahrenheit
Note that for temperature difference (change) - as used in heat loss diagrams
- 1 degree Celsius of temperature difference equals 1.8 degree Fahrenheit of temperature difference
ΔT (C°) = ΔT (°F) / 1.8 (3)
ΔT (°F) = 1.8 ΔT (C°) (4)
where
ΔT (°F) = temperature difference (°F)
ΔT (C°) = temperature difference (C°)
Example : Water is cooled from 100°C to 60 °C. What is the temperature difference in °F?
Temperature difference in degrees Celsius:
ΔT (C°) = (100 °C) - (60 °C) = 40 C°
Note that °C is used for actual temperatures (temperature relative to 273.15 K) and C° is used for temperature differences.
Temperature difference in degrees Fahrenheit calculated by using (1)
100 C° = 1.8 (100 C°) + 32 = 212 °F
60 C° = 1.8 (60 C°) + 32 = 140 °F
ΔT (°F) = 212°F - 140 °F = 72 °F
Temperature difference in degrees Fahrenheit calculated by using (3)
ΔT (°F) = 1.8 (40 C°) = 72 °F
Temperature Difference Converter
Kelvin - K
Another scale (common in science) is Kelvin, or the Absolute Temperature Scale . On the Kelvin scale the coldest temperature possible, -273 °C , has a value of 0 Kelvin (0 K) and is called the absolute zero. Units on the Kelvin scale are called Kelvins (K) and no degree symbol is used.
Because there are no lower temperatures than 0 K - the Kelvin scale does not have negative numbers.
The Kelvin has the same incremental scale as the Celsius scale and one unit Kelvin is equal in size to one unit Celsius:
1 unit Kelvin = 1 unit °C
ΔT (°K) = ΔT (°C) (5)
To calculate a Kelvin temperature, add 273 to the Celsius temperature:
T (K) = T (°C) + 273.15 (6)
Example: What is the normal body temperature of 37 oC in the Kelvin scale?
T (K) = T (°C) + 273.15 = (37 °C) + 273.15 = 310.15 K
Degree Rankine - R
In the English system the absolute temperature is in degrees Rankine (R) , not in Fahrenheit:
T (°R) = 1.8 * T (K) (7)
T (°R) = 1.8 * (T (°C) + 273.15)
T (°R) = T(°F) + 459.67 (8)
ΔT (°R) = ΔT (°F) (9)