Water - Properties at Gas-Liquid Equilibrium Conditions
Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, Prandtl number, thermal diffusivity, entropy and enthalpy).
Water tends to evaporate or vaporize by projecting molecules into the space above its surface. If the space is confined the partial pressure exerted by the molecules increases until the rate at which molecules reenter the liquid is equal to the rate at which they leave. The vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state . At higher pressures water would condense. At this equilibrium condition the vapor pressure is the saturation pressure .
The figures and tables below summarize the thermophysical properties of water and steam at equilibrium. The figures show liquid and gaseous states at equilibrium as a function of temperature, starting at the triple point and ending at the critical temperature.
Tabulated values are given below the figures, in SI and Imperial units.
Figures:
Density
Dynamic (= absolute) viscosity
Enthalpy
Entropy
Specific heat
Prandtl number
Thermal conductivity
Thermal diffusivity
Vapor pressure
Links to Unit converters
See Water and Heavy Water for thermodynamic properties at standard condtions.
See also these and other properties of Water at varying temperature and pressure : Boiling points at high pressure, Boiling points at vacuum pressure, Density and specific weight, Dynamic and kinematic viscosity, Enthalpy and entropy, Heat of vaporization, Ionization Constant, pKw , of normal and heavy water, Melting points at high pressure, Prandtl number, Properties at Gas-Liquid Equilibrium Conditions, Saturation pressure, Specific gravity, Specific heat (heat capacity), Specific volume, Thermal conductivity, Thermal diffusivity and Vapour pressure at gas-liquid equilibrium.
Vapor pressure of water as function of temperature (°C and °F):
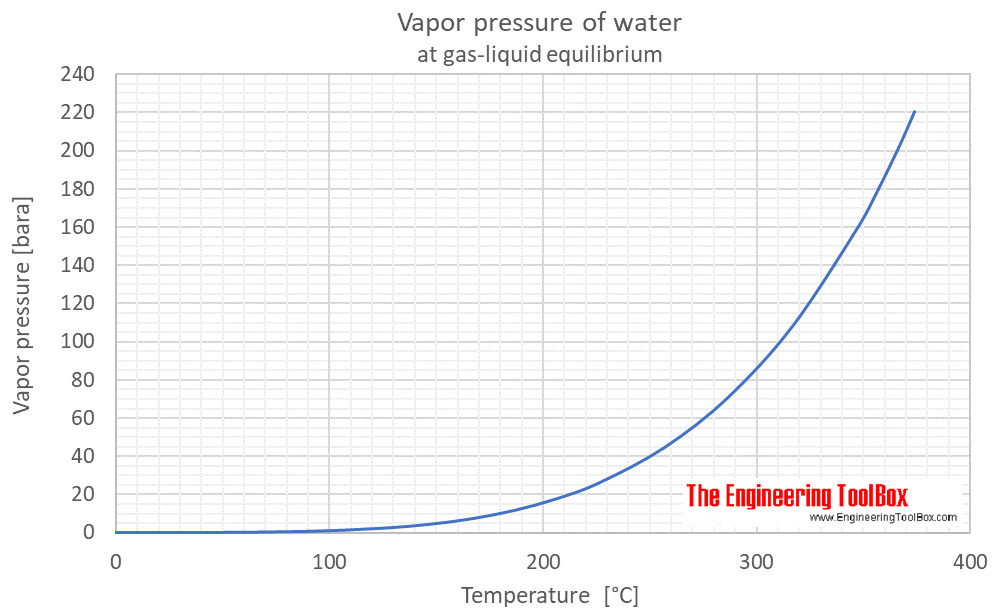
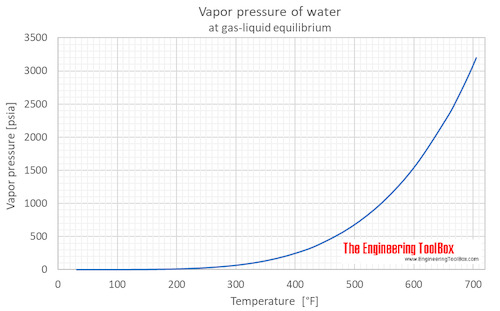
See Water - Saturation pressure for calculator of equilibrium pressure at varying temperature.
Dynamic viscosity of water (liquid and vapor) as function of temperature (°C and °F):

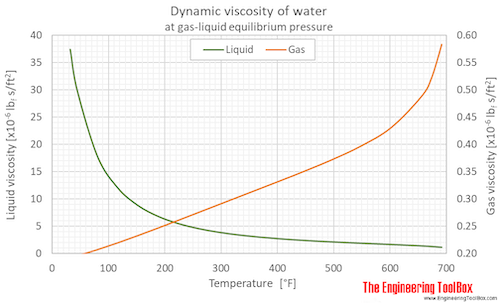
See Water - Dynamic and Kinematic Viscosity for calculator and variations at constant pressures.
Density of water (liquid and vapor) as function of temperature (°C and °F):
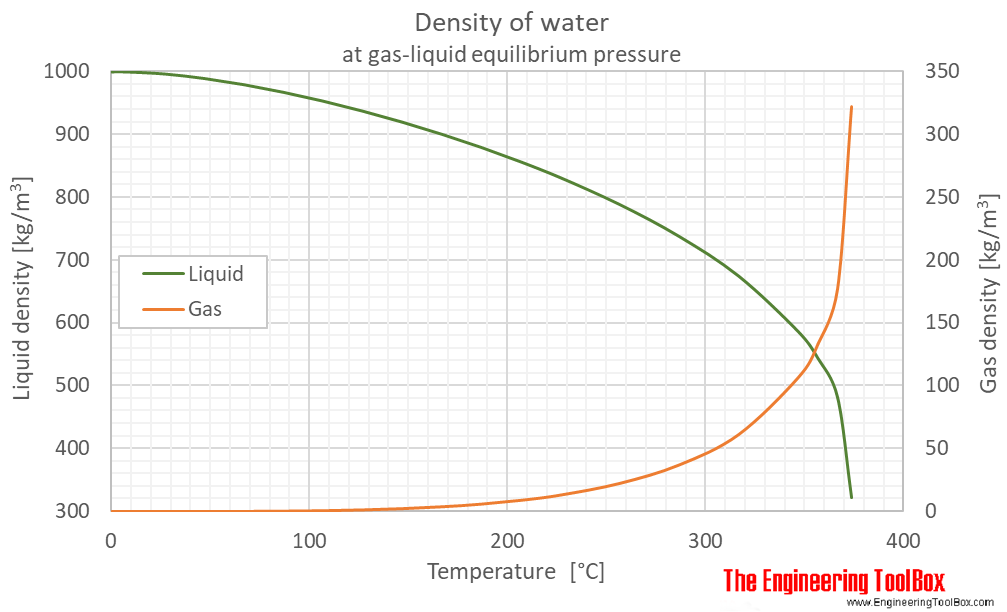
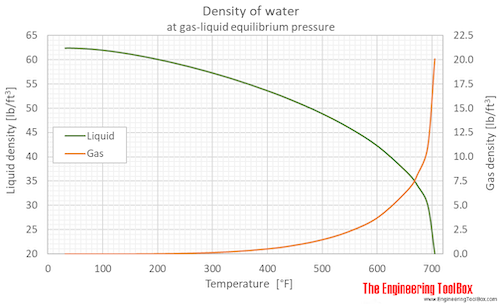
See Water - Density, Specific Weight and Thermal Expantion Coefficient for calculator and variations at constant pressures.
Specific heat (heat capacity) of water (liquid and vapor) as function of temperature (°C and °F):

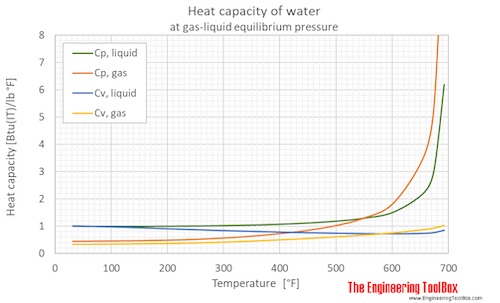
See Water - Specific heat (heat capacity) for calculator of heat capacity at given temperatures .
Thermal conductivity of water (liquid and vapor) as function of temperature (°C and °F):
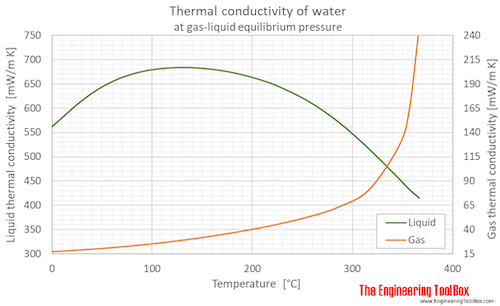
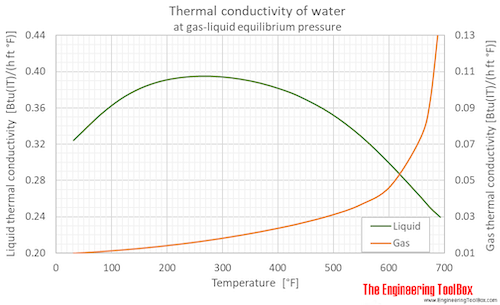 See Water - Thermal Conductivity for variations at constant pressures.
See Water - Thermal Conductivity for variations at constant pressures.
Prandtl number of water (liquid and vapor) as function of temperature (°C and °F):
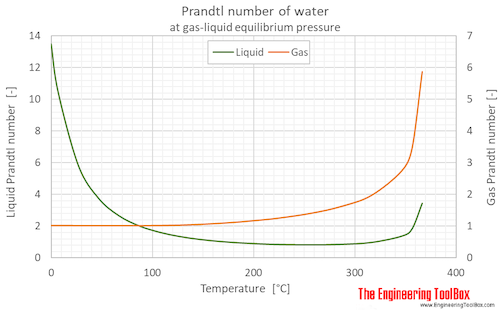
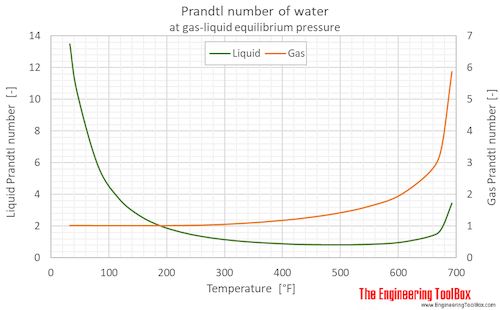
See Water - Prandtl Number for variations at constant pressures.
Thermal diffusivity of water (liquid and vapor) as function of temperature (°C and °F):
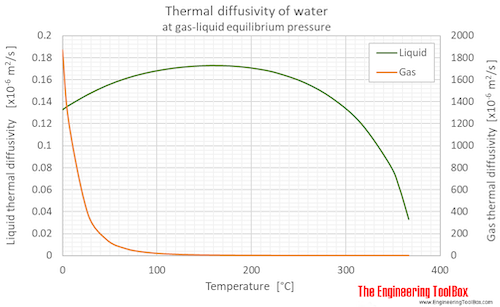
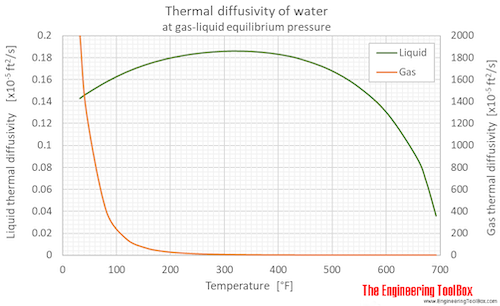
See Water - Thermal Diffusivity for variations at constant pressures.
Specific entropy of water (liquid and vapor) as function of temperature (°C and °F):
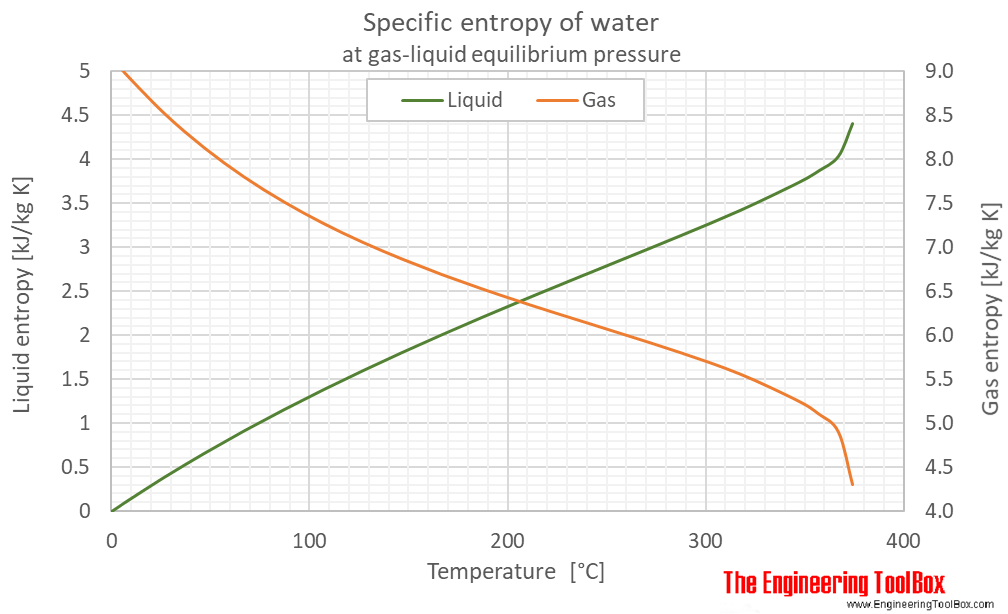
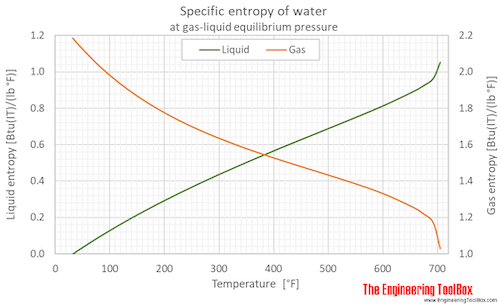 See Water - Enthalpy and Entropy for tabulated values in different units.
See Water - Enthalpy and Entropy for tabulated values in different units.
Enthalpy of water (liquid and vapor) as function of temperature (°C and °F):
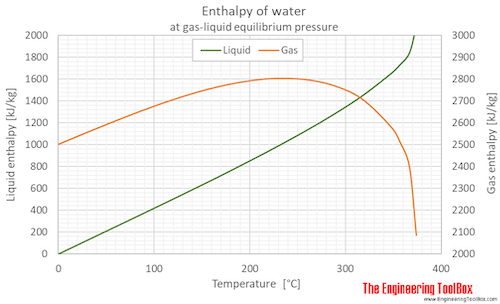
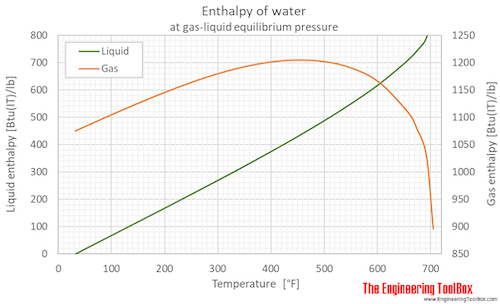 See Water - Enthalpy and Entropy for tabulated values in different units.
See Water - Enthalpy and Entropy for tabulated values in different units.
Tabulated values for water properties at gas-liquid equilibrium pressure, given in SI units:
For full table with Thermal Conductivity, Dynamic Viscosity, Enthalpy, Specific Entropy, Prandtl Number and Thermal Diffusivity - rotate the screen!
| State | Temperature | Pressure | Density | Heat Capacity CV | Heat Capacity CP | CP/CV | Thermal Conductivity | Dynamic Viscosity | Enthalpy | Specific Entropy | Prandtl Number | Thermal Diffusivity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (K) | (°C) | (bara) | (g/l), (kg/m3) | (kJ/kg K) | (kJ/kg K) | (-) | (mW/m K) | (μPa s) | (kJ/kg) | (kJ/kgK) | (-) | (×10-6 m2/s) | |
| Liquid | 273.16 | 0.01 | 6.1E-03 | 999.8 | 4.217 | 4.231 | 1.003 | 561.4 | 1791 | 6.11E-04 | 0 | 13.50 | 0.1327 |
| 280 | 6.9 | 0.0099 | 999.9 | 4.200 | 4.201 | 1.000 | 574.0 | 1434 | 28.80 | 0.1041 | 10.49 | 0.1367 | |
| 300 | 26.9 | 0.0354 | 996.5 | 4.130 | 4.181 | 1.012 | 610.3 | 853.8 | 112.6 | 0.3931 | 5.849 | 0.1465 | |
| 320 | 46.9 | 0.105 | 989.4 | 4.042 | 4.181 | 1.034 | 639.7 | 577.0 | 196.2 | 0.6628 | 3.771 | 0.1547 | |
| 340 | 66.9 | 0.272 | 979.5 | 3.942 | 4.188 | 1.063 | 660.6 | 422.0 | 279.9 | 0.9165 | 2.676 | 0.1610 | |
| 360 | 86.9 | 0.622 | 967.4 | 3.837 | 4.202 | 1.095 | 673.8 | 326.1 | 363.8 | 1.156 | 2.034 | 0.1657 | |
| 380 | 107 | 1.29 | 953.3 | 3.733 | 4.224 | 1.132 | 681.0 | 262.7 | 448.1 | 1.384 | 1.629 | 0.1691 | |
| 400 | 127 | 2.46 | 937.5 | 3.632 | 4.255 | 1.172 | 683.6 | 218.6 | 533.0 | 1.601 | 1.361 | 0.1714 | |
| 420 | 147 | 4.37 | 919.9 | 3.538 | 4.299 | 1.215 | 682.5 | 186.7 | 618.6 | 1.810 | 1.176 | 0.1726 | |
| 440 | 167 | 7.34 | 900.7 | 3.449 | 4.357 | 1.263 | 678.1 | 162.8 | 704.5 | 2.011 | 1.046 | 0.1728 | |
| 460 | 187 | 11.7 | 879.6 | 3.368 | 4.433 | 1.316 | 670.3 | 144.3 | 793.4 | 2.205 | 0.955 | 0.1719 | |
| 480 | 207 | 17.9 | 856.5 | 3.293 | 4.533 | 1.376 | 659.1 | 129.6 | 883.3 | 2.395 | 0.892 | 0.1697 | |
| 500 | 227 | 26.4 | 831.3 | 3.226 | 4.663 | 1.446 | 644.1 | 117.7 | 975.4 | 2.581 | 0.852 | 0.1661 | |
| 530 | 257 | 44.6 | 788.5 | 3.138 | 4.947 | 1.576 | 613.2 | 103.1 | 1119 | 2.856 | 0.831 | 0.1572 | |
| 560 | 287 | 71.1 | 737.8 | 3.072 | 5.424 | 1.765 | 570.2 | 90.86 | 1273 | 3.132 | 0.864 | 0.1425 | |
| 590 | 317 | 108 | 674.8 | 3.042 | 6.378 | 2.097 | 515.4 | 79.60 | 1443 | 3.418 | 0.985 | 0.1198 | |
| 620 | 347 | 159 | 586.9 | 3.114 | 9.354 | 3.004 | 454.1 | 67.38 | 1646 | 3.740 | 1.388 | 0.08272 | |
| 630 | 357 | 180 | 544.2 | 3.228 | 12.8269 | 3.974 | 432.5 | 62.24 | 1731 | 3.870 | 1.846 | 0.06196 | |
| 640 | 367 | 203 | 481.5 | 3.581 | 25.9424 | 7.244 | 414.9 | 55.25 | 1842 | 4.038 | 3.454 | 0.03322 | |
| 647.1 | 373.95 | 221 | 322.0 | 2084 | 4.407 | ||||||||
| Gas | 273.16 | 0.01 | 6.1E-03 | 0.00485 | 1.418 | 1.884 | 1.328 | 17.07 | 9.216 | 2501 | 9.156 | 1.017 | 1869 |
| 280 | 6.9 | 0.0099 | 0.00767 | 1.424 | 1.891 | 1.328 | 17.44 | 9.382 | 2513 | 8.978 | 1.017 | 1202 | |
| 300 | 26.9 | 0.0354 | 0.02558 | 1.442 | 1.914 | 1.327 | 18.67 | 9.920 | 2550 | 8.517 | 1.017 | 381.3 | |
| 320 | 46.9 | 0.105 | 0.07166 | 1.463 | 1.942 | 1.328 | 20.12 | 10.52 | 2586 | 8.130 | 1.015 | 144.6 | |
| 340 | 66.9 | 0.272 | 0.1744 | 1.489 | 1.979 | 1.329 | 21.78 | 11.16 | 2621 | 7.801 | 1.014 | 63.11 | |
| 360 | 86.9 | 0.622 | 0.3786 | 1.525 | 2.033 | 1.333 | 23.70 | 11.82 | 2654 | 7.519 | 1.014 | 30.79 | |
| 380 | 107 | 1.29 | 0.7483 | 1.575 | 2.110 | 1.339 | 25.88 | 12.50 | 2686 | 7.274 | 1.019 | 16.39 | |
| 400 | 127 | 2.46 | 1.369 | 1.643 | 2.218 | 1.350 | 28.35 | 13.19 | 2716 | 7.058 | 1.032 | 9.332 | |
| 420 | 147 | 4.37 | 2.352 | 1.734 | 2.367 | 1.365 | 31.13 | 13.88 | 2742 | 6.866 | 1.055 | 5.593 | |
| 440 | 167 | 7.34 | 3.833 | 1.845 | 2.560 | 1.388 | 34.23 | 14.57 | 2765 | 6.691 | 1.090 | 3.489 | |
| 460 | 187 | 11.7 | 5.983 | 1.974 | 2.801 | 1.419 | 37.66 | 15.26 | 2783 | 6.530 | 1.135 | 2.247 | |
| 480 | 207 | 17.9 | 9.014 | 2.117 | 3.098 | 1.463 | 41.46 | 15.95 | 2796 | 6.380 | 1.192 | 1.485 | |
| 500 | 227 | 26.4 | 13.20 | 2.271 | 3.463 | 1.525 | 45.67 | 16.65 | 2802 | 6.235 | 1.263 | 0.9991 | |
| 530 | 257 | 44.6 | 22.47 | 2.527 | 4.209 | 1.666 | 53.13 | 17.76 | 2798 | 6.024 | 1.407 | 0.5618 | |
| 560 | 287 | 71.1 | 37.15 | 2.822 | 5.410 | 1.917 | 63.34 | 19.01 | 2771 | 5.807 | 1.623 | 0.3152 | |
| 590 | 317 | 108 | 61.24 | 3.184 | 7.768 | 2.440 | 81.11 | 20.63 | 2710 | 5.565 | 1.976 | 0.1705 | |
| 620 | 347 | 159 | 106.3 | 3.674 | 14.94 | 4.067 | 126.7 | 23.37 | 2584 | 5.253 | 2.758 | 0.07973 | |
| 630 | 357 | 180 | 132.8 | 3.911 | 22.66 | 5.793 | 163.4 | 25.02 | 2511 | 5.108 | 3.468 | 0.05430 | |
| 640 | 367 | 203 | 177.1 | 4.306 | 52.59 | 12.21 | 250.0 | 27.94 | 2396 | 4.903 | 5.876 | 0.02684 | |
| 647.1 | 373.95 | 221 | 322.0 | 2084 | 4.306 | ||||||||
Tabulated values for water properties at gas-liquid equilibrium pressure, given in Imperial units:
For full table with Thermal Conductivity, Dynamic Viscosity, Enthalpy, Specific Entropy, Prandtl Number and Thermal Diffusivity -- rotate the screen!
| State | Temperature | Pressure | Density | Heat Capacity CV | Heat Capacity CP | CP/CV | Thermal Conductivity | Dynamic Viscosity | Enthalpy | Specific Entropy | Prandtl Number | Thermal Diffusivity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (K) | (°F) | (psia) | (lb/ft3) | (Btu(IT)/lb °F) | (Btu(IT)/lb °F) | (-) | (Btu(IT)/h ft °F) | (×10-6 lbf s/ft2) | (×10-6 lbm s/ft s) | (Btu(IT)/lb) | (Btu(IT)/(lb °F)) | (-) | (×10-5ft2/s) | |
| Liquid | 273.16 | 32.018 | 0.0888 | 62.42 | 1.007 | 1.011 | 1.003 | 0.3244 | 37.41 | 1204 | 2.63E-04 | 0 | 13.50 | 0.1428 |
| 280 | 44.3 | 0.144 | 62.42 | 1.003 | 1.003 | 1.000 | 0.3317 | 29.94 | 963.4 | 12.38 | 0.02487 | 10.49 | 0.1471 | |
| 300 | 80.3 | 0.513 | 62.21 | 0.9865 | 0.9986 | 1.012 | 0.3526 | 17.83 | 573.8 | 48.39 | 0.09389 | 5.849 | 0.1577 | |
| 320 | 116 | 1.53 | 61.77 | 0.9653 | 0.9985 | 1.034 | 0.3696 | 12.05 | 387.7 | 84.34 | 0.1583 | 3.771 | 0.1665 | |
| 340 | 152 | 3.94 | 61.15 | 0.9414 | 1.000 | 1.063 | 0.3817 | 8.813 | 283.6 | 120.3 | 0.2189 | 2.676 | 0.1733 | |
| 360 | 188 | 9.02 | 60.39 | 0.9164 | 1.004 | 1.095 | 0.3893 | 6.811 | 219.1 | 156.4 | 0.2762 | 2.034 | 0.1784 | |
| 380 | 224 | 18.7 | 59.51 | 0.8916 | 1.009 | 1.132 | 0.3935 | 5.486 | 176.5 | 192.6 | 0.3306 | 1.629 | 0.1820 | |
| 400 | 260 | 35.6 | 58.52 | 0.8676 | 1.016 | 1.172 | 0.3950 | 4.566 | 146.9 | 229.1 | 0.3825 | 1.361 | 0.1845 | |
| 420 | 296 | 63.4 | 57.43 | 0.8449 | 1.027 | 1.215 | 0.3944 | 3.899 | 125.4 | 265.9 | 0.4322 | 1.176 | 0.1858 | |
| 440 | 332 | 106 | 56.23 | 0.8238 | 1.041 | 1.263 | 0.3918 | 3.400 | 109.4 | 302.9 | 0.4802 | 1.046 | 0.1860 | |
| 460 | 368 | 170 | 54.91 | 0.8044 | 1.059 | 1.316 | 0.3873 | 3.014 | 96.97 | 341.1 | 0.5267 | 0.955 | 0.1850 | |
| 480 | 404 | 260 | 53.47 | 0.7866 | 1.083 | 1.376 | 0.3808 | 2.708 | 87.11 | 379.8 | 0.5721 | 0.892 | 0.1827 | |
| 500 | 440 | 383 | 51.90 | 0.7704 | 1.114 | 1.446 | 0.3721 | 2.457 | 79.06 | 419.4 | 0.6165 | 0.852 | 0.1788 | |
| 530 | 494 | 646 | 49.23 | 0.7495 | 1.182 | 1.576 | 0.3543 | 2.152 | 69.25 | 481.2 | 0.6822 | 0.831 | 0.1692 | |
| 560 | 548 | 1031 | 46.06 | 0.7338 | 1.295 | 1.765 | 0.3295 | 1.898 | 61.05 | 547.3 | 0.7481 | 0.864 | 0.1534 | |
| 590 | 602 | 1569 | 42.13 | 0.7266 | 1.523 | 2.097 | 0.2978 | 1.662 | 53.49 | 620.4 | 0.8164 | 0.985 | 0.1289 | |
| 620 | 656 | 2306 | 36.64 | 0.7438 | 2.234 | 3.004 | 0.2624 | 1.407 | 45.28 | 707.5 | 0.8932 | 1.388 | 0.08903 | |
| 630 | 674 | 2606 | 33.98 | 0.7710 | 3.064 | 3.974 | 0.2499 | 1.300 | 41.83 | 744.1 | 0.9243 | 1.846 | 0.06669 | |
| 640 | 692 | 2939 | 30.06 | 0.8554 | 6.196 | 7.244 | 0.2397 | 1.154 | 37.12 | 791.8 | 0.9644 | 3.454 | 0.03575 | |
| 647.1 | 705.11 | 3200 | 20.10 | 896.1 | 1.053 | |||||||||
| Gas | 273.16 | 32.018 | 0.0888 | 3.03E-04 | 0.3388 | 0.4501 | 1.328 | 0.00986 | 0.1925 | 6.193 | 1075 | 2.187 | 1.017 | 2012 |
| 280 | 44.3 | 0.144 | 4.79E-04 | 0.3402 | 0.4517 | 1.328 | 0.01008 | 0.1959 | 6.304 | 1081 | 2.144 | 1.017 | 1293 | |
| 300 | 80.3 | 0.513 | 0.00160 | 0.3445 | 0.4572 | 1.327 | 0.01079 | 0.2072 | 6.666 | 1096 | 2.034 | 1.017 | 410.5 | |
| 320 | 116 | 1.53 | 0.00447 | 0.3493 | 0.4638 | 1.328 | 0.01162 | 0.2197 | 7.068 | 1112 | 1.942 | 1.015 | 155.6 | |
| 340 | 152 | 3.94 | 0.01089 | 0.3556 | 0.4727 | 1.329 | 0.01259 | 0.2330 | 7.497 | 1127 | 1.863 | 1.014 | 67.93 | |
| 360 | 188 | 9.02 | 0.02363 | 0.3642 | 0.4855 | 1.333 | 0.01369 | 0.2469 | 7.945 | 1141 | 1.796 | 1.014 | 33.15 | |
| 380 | 224 | 18.7 | 0.04671 | 0.3762 | 0.5039 | 1.339 | 0.01495 | 0.2612 | 8.402 | 1155 | 1.737 | 1.019 | 17.64 | |
| 400 | 260 | 35.6 | 0.08549 | 0.3925 | 0.5298 | 1.350 | 0.01638 | 0.2755 | 8.865 | 1168 | 1.686 | 1.032 | 10.04 | |
| 420 | 296 | 63.4 | 0.1468 | 0.4140 | 0.5652 | 1.365 | 0.01799 | 0.2900 | 9.329 | 1179 | 1.640 | 1.055 | 6.020 | |
| 440 | 332 | 106 | 0.2393 | 0.4406 | 0.6114 | 1.388 | 0.01978 | 0.3044 | 9.793 | 1189 | 1.598 | 1.090 | 3.755 | |
| 460 | 368 | 170 | 0.3735 | 0.4715 | 0.6691 | 1.419 | 0.02176 | 0.3187 | 10.25 | 1196 | 1.560 | 1.135 | 2.419 | |
| 480 | 404 | 260 | 0.5627 | 0.5056 | 0.7399 | 1.463 | 0.02395 | 0.3332 | 10.72 | 1202 | 1.524 | 1.192 | 1.598 | |
| 500 | 440 | 383 | 0.8240 | 0.5425 | 0.8271 | 1.525 | 0.02639 | 0.3478 | 11.19 | 1205 | 1.489 | 1.263 | 1.075 | |
| 530 | 494 | 646 | 1.403 | 0.6035 | 1.005 | 1.666 | 0.03070 | 0.3708 | 11.93 | 1203 | 1.439 | 1.407 | 0.6047 | |
| 560 | 548 | 1031 | 2.319 | 0.6741 | 1.292 | 1.917 | 0.03660 | 0.3970 | 12.77 | 1191 | 1.387 | 1.623 | 0.3393 | |
| 590 | 602 | 1569 | 3.823 | 0.7605 | 1.855 | 2.440 | 0.04686 | 0.4310 | 13.87 | 1165 | 1.329 | 1.976 | 0.1835 | |
| 620 | 656 | 2306 | 6.636 | 0.8776 | 3.569 | 4.067 | 0.07318 | 0.4882 | 15.71 | 1111 | 1.255 | 2.758 | 0.08582 | |
| 630 | 674 | 2606 | 8.293 | 0.9342 | 5.412 | 5.793 | 0.09443 | 0.5225 | 16.81 | 1080 | 1.220 | 3.468 | 0.05845 | |
| 640 | 692 | 2939 | 11.06 | 1.028 | 12.56 | 12.21 | 0.1445 | 0.5835 | 18.77 | 1030 | 1.171 | 5.876 | 0.02889 | |
| 647.1 | 705.11 | 3200 | 20.10 | 896.1 | 1.029 | |||||||||
Density unit converter
Dynamic viscosity unit converter
Kinematic viscosity unit converter
Pressure unit converter
Specific heat (heat capacity) and entropy unit converter
Temperature unit converter
Thermal conductivity unit converter
Back to top



