Moist Air - Water Vapor and Saturation Pressure
Saturation pressure of water vapor in moist air vs. temperature.
Water vapor is almost always present in the surrounding air.
Note! - the equations below is for pure water vapor - not moist air .
Saturation Pressure of Water Vapor
The maximum saturation pressure of the water vapor in moist air varies with the temperature of the air vapor mixture and can be expressed as:
p ws = e (77.3450 + 0.0057 T - 7235 / T) / T 8.2 (1)
where
p ws = water vapor saturation pressure (Pa)
e = the constant 2.718.......
T = dry bulb temperature of the moist air (K)
Density of Water Vapor
The density of water vapor can be expressed as:
ρ w = 0.0022 p w / T (2)
where
p w = partial pressure water vapor (Pa, N/m2)
ρ w = density water vapor (kg/m3 )
T = absolute dry bulb temperature (K)
Saturation pressure and density of water vapor for common temperatures
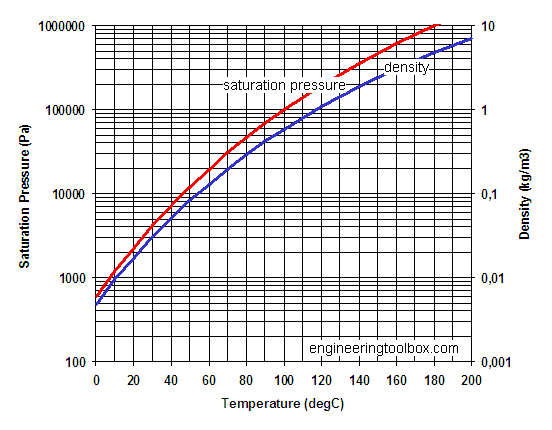
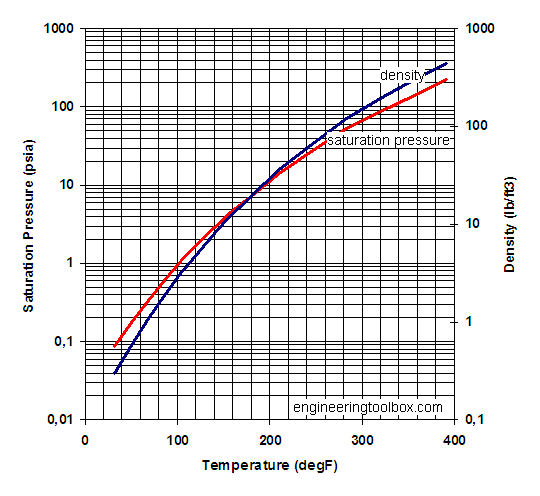
| Temperature | Saturation Pressure | Density | |||||
|---|---|---|---|---|---|---|---|
| (oC) | ( oF) | (Pa) | (mmHg) | (psia) | (inHg) | (kg/m3 ) | 10-3 (lb/ft3 ) |
| 0 | 32 | 603 | 4.6 | 0.09 | 0.18 | 0.005 | 0.30 |
| 10 | 50 | 1212 | 9.2 | 0.18 | 0.36 | 0.009 | 0.59 |
| 20 | 68 | 2310 | 17.4 | 0.33 | 0.68 | 0.017 | 1.08 |
| 30 | 86 | 4195 | 31.7 | 0.61 | 1.24 | 0.030 | 1.90 |
| 40 | 104 | 7297 | 55.1 | 1.06 | 2.15 | 0.051 | 3.20 |
| 50 | 122 | 12210 | 92.2 | 1.8 | 3.60 | 0.083 | 5.19 |
| 60 | 140 | 19724 | 149 | 2.9 | 5.82 | 0.13 | 8.13 |
| 70 | 158 | 30866 | 233 | 4.5 | 9.11 | 0.20 | 12.3 |
| 80 | 176 | 46925 | 354 | 6.8 | 13.8 | 0.29 | 18.2 |
| 90 | 194 | 69485 | 525 | 10.1 | 20.5 | 0.42 | 26.3 |
| 100 | 212 | 100446 | 758 | 14.6 | 29.6 | 0.59 | 36.9 |
| 120 | 248 | 196849 | 1486 | 28.6 | 58.1 | 1.10 | 68.7 |
| 140 | 284 | 358137 | 2704 | 51.9 | 105.7 | 1.91 | 119 |
| 160 | 320 | 611728 | 4619 | 88.7 | 180.5 | 3.11 | 194 |
| 180 | 356 | 990022 | 7475 | 144 | 292.1 | 4.80 | 300 |
| 200 | 392 | 1529627 | 11549 | 222 | 451.2 | 7.11 | 444 |
- 1 lb/ft3 = 16.018 kg/m3
- 1 kg/m3 = 0.0624 lb/ft3
Example - Saturation Pressure Water Vapor
The Saturation pressure of water vapor in moist air at dry bulb temperature 25 oC can be calculated:
First, convertion from °C to K:
( 25 °C) + 273 = 298 (K)
Then Eq. (1) is used:
p ws = e (77.3450 + 0.0057 (298 K) - 7235 / (298 K) ) / 298 [K] 8.2
= 3130 (Pa)
Related Topics
-
Air Psychrometrics
Moist and humid air calculations. Psychrometric charts and Mollier diagrams. Air-condition systems temperatures, absolute and relative humidities and moisture content in air.
Related Documents
-
Air - Drying Force
The drying force of air depends on the air moisture holding capacity and the water surface to air evaporation capacity. -
Air - Humidity Measurement from Dry and Wet Bulb Temperature
Relative humidity in moist air can estimated by measuring the dry and wet bulb temperature. -
Air - Maximum Moisture Carrying Capacity
Maximum water content in humid air vs. temperature. -
Air - Moisture Holding Capacity vs. Temperature
The moisture holding capacity of air increases with temperature. -
Dry Air - Thermodynamic and Physical Properties
Thermodynamic properties of dry air - specific heat, ratio of specific heats, dynamic viscosity, thermal conductivity, Prandtl number, density and kinematic viscosity at temperatures ranging 175 - 1900 K. -
Humid Air and the Ideal Gas Law
Pressure, temperature and volume in a perfect ideal gas like moist air (air with water vapor). -
Liquids - Vapor Pressures
Vapor and saturation pressure for some common liquids. -
Moist Air - Degree of Saturation
Humidity ratio of moist air to humidity ratio of saturated moist air. -
Moist Air - Density vs. Pressure
Density of moist air vs. pressure ranging 75 - 1000 mmHg. -
Moist Air - Density vs. Water Content and Temperature
Density of the mix of dry air and water vapor - moist humid air. -
Moist Air - Mole Fraction of Water Vapor
Mole fraction of water vapor is the ratio of water molecules to air and water molecules. -
Moist Air - Partial Pressure and Daltons Law
The pressure in a mixture of dry air and water vapor - humid or moist air - can be estimated by using Daltons Law of partial pressures. -
Moist Air - Psychrometric Table for Pressure 29.92 inHg
Dry and wet bulb temperatures, saturation pressure, water vapor weight, specific volume, heat and more. -
Moist Air - Vapor Pressure
Vapor pressures vs. dry and wet bulb temperatures in moist air. -
Removing Heat with Air
Calculating heat removed with air by measuring the wet bulb temperature. -
Water - Boiling Points at Vacuum Pressure
Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, SI and Imperial units. -
Water - Saturation Pressure and Specific Weight vs. Temperature
Vapor pressure and specific weight of water at temperatures ranging 32 to 212 oF - Imperial Units. -
Water - Saturation Pressure vs. Temperature
Online calculator, figures and tables with water saturation (vapor) pressure at temperatures ranging 0 to 370 °C (32 to 700°F) - in Imperial and SI Units. -
Wet Bulb Globe Temperature (WBGT)
The Wet Bulb Globe Temperature can be used to measure the general Heat-Stress index.




