Butane - Thermal Conductivity vs. Temperature and Pressure
Online calculators, figures and tables showing thermal conductivity of liquid and gaseous butane, C4H10, at varying temperature and pressure, SI and Imperial units.
Thermal conductivity is a material property that describes ability to conduct heat. Thermal conductivity can be defined as
"the quantity of heat transmitted through a unit thickness of a material - in a direction normal to a surface of unit area - due to a unit temperature gradient under steady state conditions"
The SI unit used for thermal conductivity is (W/m K), while a common Imperial unit is (Btu/h ft °F).
Thermal conductivity unit converter
The thermal conductivity of butane depends on temperature and pressure as shown in the figures and tables below.
Online Butane Thermal Conductivity Calculator
The calculator below can be used to estimate the thermal conductivity of butane at given temperature and atmospheric pressure.
Note! Butane boiling point at atmospheric pressure is -0.8°C / 30.5°F, and hence, there is a shift in conductivity at that temperature.
The output thermal conductivity is given as mW/(m K), Btu(IT)/(h ft °F), (Btu(IT) in)/(h ft2°F) and kcal(IT)/(h m K).
See also other properties of Butane at varying temperature and pressure: Density and specific weight, Dynamic and Kinematic Viscosity, Specific Heat (Heat Capacity) and Thermophysical properties at standard conditions,
as well as thermal conductivity o f air, ammonia, butane, carbon dioxide, ethane, ethylene, hydrogen, methane, nitrogen, propane and water . For thermal conductivity of construction materials, see related documents at the bottom of the page.
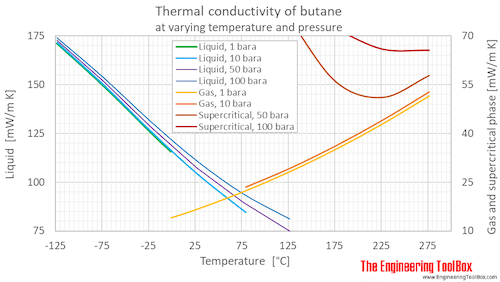
Thermal conductivity of butane at given temperatures (°C and °F) and 1 bara:
For full table with Thermal Conductivity - rotate the screen!
| State | Temperature | Thermal conductivity | Temperature | Thermal conductivity | |||||
|---|---|---|---|---|---|---|---|---|---|
| (K) | (°C) | (mW/m K) | (kcal(IT)/(h m K)) | (K) | (°F) | (Btu(IT)/(h ft °F)) | (kcal(IT)/(h m K)) | ||
| Liquid | 78.2 | -195 | 192.1 | 0.1652 | 77.6 | -320 | 0.1110 | 0.1653 | |
| 123 | -150 | 180.9 | 0.1556 | 116 | -250 | 0.1057 | 0.1574 | ||
| 148 | -125 | 171.9 | 0.1478 | 144 | -200 | 0.1002 | 0.1491 | ||
| 173 | -100 | 161.6 | 0.1389 | 172 | -150 | 0.09363 | 0.1393 | ||
| 198 | -75 | 150.3 | 0.1292 | 200 | -100 | 0.08637 | 0.1285 | ||
| 223 | -50 | 138.5 | 0.1191 | 228 | -50 | 0.07879 | 0.1173 | ||
| 231 | -42 | 134.7 | 0.1158 | 231 | -44 | 0.07788 | 0.1159 | ||
| 248 | -25 | 126.7 | 0.1089 | 244 | -20 | 0.07424 | 0.1105 | ||
| 263 | -10 | 119.8 | 0.1030 | 255 | 0 | 0.07126 | 0.1060 | ||
| 268 | -5 | 117.5 | 0.1011 | 266 | 20 | 0.06834 | 0.1017 | ||
| 272.3 | -0.84 | 115.7 | 0.09947 | 272.3 | 30.49 | 0.06684 | 0.09947 | ||
| Gas | 272.3 | -0.84 | 14.11 | 0.01214 | 272.3 | 30.49 | 0.00815 | 0.01214 | |
| 283 | 10 | 15.12 | 0.01300 | 283 | 50 | 0.00873 | 0.01300 | ||
| 293 | 20 | 16.07 | 0.01382 | 300 | 80 | 0.00967 | 0.01438 | ||
| 303 | 30 | 17.06 | 0.01467 | 305 | 90 | 0.00999 | 0.01486 | ||
| 323 | 50 | 19.13 | 0.01645 | 311 | 100 | 0.01032 | 0.01535 | ||
| 348 | 75 | 21.90 | 0.01883 | 339 | 150 | 0.01204 | 0.01791 | ||
| 373 | 100 | 24.87 | 0.02138 | 366 | 200 | 0.01390 | 0.02069 | ||
| 398 | 125 | 28.03 | 0.02410 | 394 | 250 | 0.01591 | 0.02367 | ||
| 423 | 150 | 31.40 | 0.02700 | 422 | 300 | 0.01805 | 0.02686 | ||
| 448 | 175 | 34.96 | 0.03006 | 478 | 400 | 0.02277 | 0.03389 | ||
| 473 | 200 | 38.72 | 0.03329 | 533 | 500 | 0.02806 | 0.04176 | ||
| 523 | 250 | 46.84 | 0.04028 | 589 | 600 | 0.03392 | 0.05047 | ||
| 573 | 300 | 55.76 | 0.04795 | 644 | 700 | 0.04034 | 0.06004 | ||
| 623 | 350 | 65.48 | 0.05630 | 700 | 800 | 0.04734 | 0.07045 | ||
| 673 | 400 | 76.00 | 0.06535 | 755 | 900 | 0.05491 | 0.08171 | ||
| 773 | 500 | 99.42 | 0.08549 | 811 | 1000 | 0.06303 | 0.09380 | ||
Thermal conductivity unit converter
Thermal conductivity of butane at given temperatures and pressures, SI and Imperial units:
For full table with Thermal Conductivity - rotate the screen!
| State | Temperature | Pressure | Thermal conductivity | |||||
|---|---|---|---|---|---|---|---|---|
| (K) | (°C) | (°F) | (bara) | (psia) | (mW/m K) | (kcal(IT)/(h m K)) | (Btu(IT)/(h ft °F)) | |
| Liquid at equilibrium | 134.9 | -138.25 | -216.85 | 6.7E-06 | 9.7E-05 | 176.6 | 0.1518 | 0.1020 |
| 150 | -123 | -190 | 8.6E-05 | 1.2E-03 | 171.2 | 0.1472 | 0.09892 | |
| 200 | -73 | -100 | 0.0194 | 0.281 | 149.4 | 0.1285 | 0.08632 | |
| 220 | -53 | -64 | 0.0781 | 1.13 | 139.9 | 0.1203 | 0.08083 | |
| 240 | -33.2 | -28 | 0.241 | 3.49 | 130.4 | 0.1121 | 0.07534 | |
| 260 | -13.2 | 8.3 | 0.610 | 8.84 | 121.2 | 0.1042 | 0.07003 | |
| 280 | 6.9 | 44.3 | 1.33 | 19.3 | 112.3 | 0.09656 | 0.06489 | |
| 300 | 26.9 | 80.3 | 2.58 | 37.4 | 103.9 | 0.08934 | 0.06003 | |
| 320 | 46.9 | 116 | 4.56 | 66.2 | 96.10 | 0.08263 | 0.05553 | |
| 340 | 66.9 | 152 | 7.52 | 109 | 88.88 | 0.07642 | 0.05135 | |
| 360 | 86.9 | 188 | 11.7 | 170 | 82.27 | 0.07074 | 0.04753 | |
| 380 | 106.9 | 224 | 17.4 | 252 | 76.22 | 0.06554 | 0.04404 | |
| 400 | 126.9 | 260 | 25.0 | 362 | 70.60 | 0.06071 | 0.04079 | |
| 420 | 146.9 | 296 | 34.9 | 506 | 67.19 | 0.05777 | 0.03882 | |
| 134.9 | -138.25 | -216.85 | 6.7E-06 | 9.7E-05 | 4.855 | 0.00417 | 0.00281 | |
| Gas at equilibrium | 150 | -123 | -190 | 8.6E-05 | 1.2E-03 | 5.579 | 0.00480 | 0.00322 |
| 200 | -73 | -100 | 0.0194 | 0.281 | 8.497 | 0.00731 | 0.00491 | |
| 220 | -53 | -64 | 0.0781 | 1.13 | 9.884 | 0.00850 | 0.00571 | |
| 240 | -33.2 | -28 | 0.241 | 3.49 | 11.39 | 0.00979 | 0.00658 | |
| 260 | -13.2 | 8.3 | 0.610 | 8.84 | 13.03 | 0.01120 | 0.00753 | |
| 280 | 6.9 | 44.3 | 1.33 | 19.3 | 14.82 | 0.01274 | 0.00856 | |
| 300 | 26.9 | 80.3 | 2.58 | 37.4 | 16.78 | 0.01443 | 0.00970 | |
| 320 | 46.9 | 116 | 4.56 | 66.2 | 19.00 | 0.01634 | 0.01098 | |
| 340 | 66.9 | 152 | 7.52 | 109 | 21.58 | 0.01856 | 0.01247 | |
| 360 | 86.9 | 188 | 11.7 | 170 | 24.72 | 0.02126 | 0.01428 | |
| 380 | 107 | 224 | 17.4 | 252 | 28.81 | 0.02477 | 0.01665 | |
| 400 | 127 | 260 | 25.0 | 362 | 35.03 | 0.03012 | 0.02024 | |
| 420 | 147 | 296 | 34.9 | 506 | 53.10 | 0.04566 | 0.03068 | |
| Liquid | 150 | -123 | -190 | 1 | 14.5 | 171.2 | 0.1472 | 0.09893 |
| 200 | -73.2 | -100 | 1 | 14.5 | 149.4 | 0.1285 | 0.08632 | |
| 250 | -23.2 | -9.7 | 1 | 14.5 | 125.8 | 0.1082 | 0.07269 | |
| 272.31 | -0.84 | 30.49 | 1 | 14.5 | 115.7 | 0.09947 | 0.06684 | |
| Gas | 272.31 | -0.84 | 30.49 | 1 | 14.5 | 14.11 | 0.01213 | 0.00815 |
| 300 | 26.9 | 80.3 | 1 | 14.5 | 16.75 | 0.01440 | 0.00968 | |
| 350 | 76.9 | 170 | 1 | 14.5 | 22.12 | 0.01902 | 0.01278 | |
| 400 | 127 | 260 | 1 | 14.5 | 28.28 | 0.02431 | 0.01634 | |
| 450 | 177 | 350 | 1 | 14.5 | 35.23 | 0.03029 | 0.02036 | |
| 500 | 227 | 440 | 1 | 14.5 | 42.98 | 0.03696 | 0.02483 | |
| 550 | 277 | 530 | 1 | 14.5 | 51.53 | 0.04431 | 0.02978 | |
| Liquid | 150 | -123 | -190 | 10 | 145 | 171.5 | 0.1474 | 0.09908 |
| 200 | -73.2 | -100 | 10 | 145 | 149.8 | 0.1288 | 0.08655 | |
| 250 | -23.2 | -9.7 | 10 | 145 | 126.3 | 0.1086 | 0.07300 | |
| 300 | 26.9 | 80.3 | 10 | 145 | 104.5 | 0.08986 | 0.06038 | |
| 350 | 76.9 | 170 | 10 | 145 | 85.56 | 0.07357 | 0.04944 | |
| 352.62 | 79.47 | 175.05 | 10 | 145 | 84.64 | 0.07277 | 0.04890 | |
| Gas | 352.62 | 79.47 | 175.05 | 10 | 145 | 23.47 | 0.02018 | 0.01356 |
| 400 | 127 | 260 | 10 | 145 | 29.23 | 0.02513 | 0.01689 | |
| 450 | 177 | 350 | 10 | 145 | 36.24 | 0.03116 | 0.02094 | |
| 500 | 227 | 440 | 10 | 145 | 44.07 | 0.03789 | 0.02546 | |
| 550 | 277 | 530 | 10 | 145 | 52.70 | 0.04532 | 0.03045 | |
| Liquid | 150 | -123 | -190 | 50 | 725 | 172.6 | 0.1484 | 0.09974 |
| 200 | -73.2 | -100 | 50 | 725 | 151.5 | 0.1303 | 0.08755 | |
| 250 | -23.2 | -9.7 | 50 | 725 | 128.6 | 0.1106 | 0.07433 | |
| 300 | 26.9 | 80.3 | 50 | 725 | 107.5 | 0.09243 | 0.06211 | |
| 350 | 76.9 | 170 | 50 | 725 | 89.62 | 0.07706 | 0.05178 | |
| 400 | 127 | 260 | 50 | 725 | 75.04 | 0.06452 | 0.04335 | |
| Supercritical phase |
450 | 177 | 350 | 50 | 725 | 55.86 | 0.04803 | 0.03228 |
| 500 | 227 | 440 | 50 | 725 | 51.14 | 0.04397 | 0.02955 | |
| 550 | 277 | 530 | 50 | 725 | 57.84 | 0.04973 | 0.03342 | |
| Liquid | 150 | -123 | -190 | 100 | 1450 | 174.0 | 0.1496 | 0.1006 |
| 200 | -73.2 | -100 | 100 | 1450 | 153.6 | 0.1321 | 0.08875 | |
| 250 | -23.2 | -9.7 | 100 | 1450 | 131.4 | 0.1130 | 0.07592 | |
| 300 | 26.9 | 80.3 | 100 | 1450 | 111.0 | 0.09543 | 0.06413 | |
| 350 | 76.9 | 170 | 100 | 1450 | 94.02 | 0.08084 | 0.05432 | |
| 400 | 127 | 260 | 100 | 1450 | 81.08 | 0.06972 | 0.04685 | |
| Supercritical phase |
450 | 177 | 350 | 100 | 1450 | 71.91 | 0.06183 | 0.04155 |
| 500 | 227 | 440 | 100 | 1450 | 65.91 | 0.05667 | 0.03808 | |
| 550 | 277 | 530 | 100 | 1450 | 65.62 | 0.05642 | 0.03791 | |
| Liquid | 150 | -123 | -190 | 300 | 4351 | 179.3 | 0.1542 | 0.1036 |
| 200 | -73.2 | -100 | 300 | 4351 | 161.4 | 0.1388 | 0.09324 | |
| 250 | -23.2 | -9.7 | 300 | 4351 | 141.4 | 0.1216 | 0.08172 | |
| 300 | 26.9 | 80.3 | 300 | 4351 | 123.1 | 0.1058 | 0.07112 | |
| 350 | 76.9 | 170 | 300 | 4351 | 108.1 | 0.09292 | 0.06244 | |
| 400 | 127 | 260 | 300 | 4351 | 97.05 | 0.08345 | 0.05608 | |
| Supercritical phase |
450 | 177 | 350 | 300 | 4351 | 90.03 | 0.07741 | 0.05202 |
| 500 | 227 | 440 | 300 | 4351 | 86.54 | 0.07441 | 0.05000 | |
| 550 | 277 | 530 | 300 | 4351 | 85.90 | 0.07386 | 0.04963 | |
| Liquid | 150 | -123 | -190 | 650 | 9427 | 187.4 | 0.1612 | 0.1083 |
| 200 | -73.2 | -100 | 650 | 9427 | 173.2 | 0.1489 | 0.1001 | |
| 250 | -23.2 | -9.7 | 650 | 9427 | 156.3 | 0.1344 | 0.09033 | |
| 300 | 26.9 | 80.3 | 650 | 9427 | 140.3 | 0.1207 | 0.08109 | |
| 350 | 76.9 | 170 | 650 | 9427 | 127.0 | 0.1092 | 0.07336 | |
| 400 | 127 | 260 | 650 | 9427 | 116.9 | 0.1005 | 0.06754 | |
| Supercritical phase |
450 | 177 | 350 | 650 | 9427 | 110.2 | 0.09474 | 0.06366 |
| 500 | 227 | 440 | 650 | 9427 | 106.6 | 0.09162 | 0.06156 | |
| 550 | 277 | 530 | 650 | 9427 | 105.6 | 0.09079 | 0.06101 | |



