Methane - Thermal Conductivity vs. Temperature and Pressure
Online calculator, figures and table showing thermal conductivity of methane, CH4, at temperatures ranging from -160 to 725 °C (-260 to 1300 °F) at atmospheric and higher pressure - Imperial and SI Units.
Thermal conductivity is a material property that describes ability to conduct heat . Thermal conductivity can be defined as
" the quantity of heat transmitted through a unit thickness of a material - in a direction normal to a surface of unit area - due to a unit temperature gradient under steady state conditions".
Thermal conductivity most common units are W/(m K) in the SI system and Btu/(h ft °F) in the Imperial system.
Tabulated values and thermal conductivity units conversion are given below the figures.
Online Methane Thermal Conductivity Calculator
The calculator below can be used to estimate the thermal conductivity of gaseous methane at given temperatures and 1 bara.
The output conductivity is given as mW/(m K), Btu(IT)/(h ft °F), (Btu(IT) in)/(h ft2°F) and kcal(IT)/(h m K).
See also other properties of Methane at varying temperature and pressure : Density and specific weight, Dynamic and kinematic viscosity, Prandtl number and Specific heat (Heat capacity), and Thermophysical properties at standard conditions,
as well as thermal conductivity o f air, ammonia, butane, carbon dioxide, ethane, ethylene, hydrogen, nitrogen, propane and water.
See also Conductive Heat Transfer Calculator
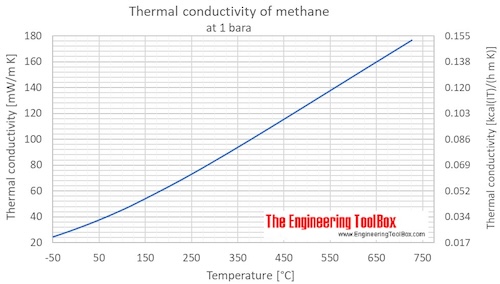
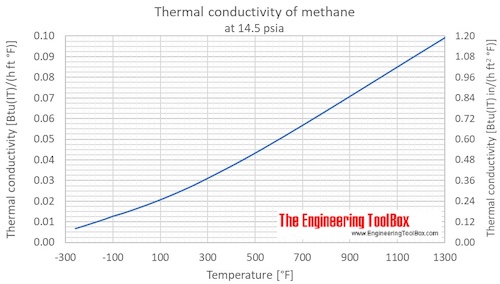
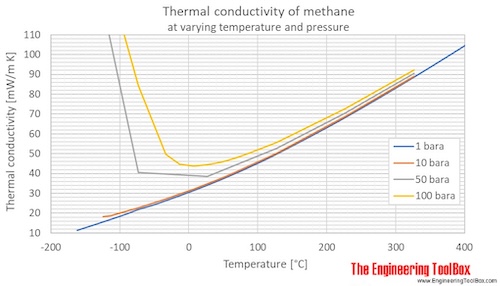
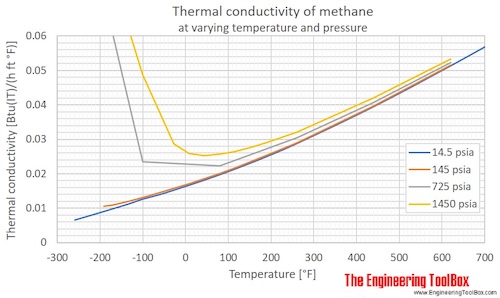
Thermal conductivity of methane at given temperatures and pressures:
For full table with Thermal Conductivity - rotate the screen!
| State | Temperature | Pressure | Thermal conductivity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (K) | (°C) | (°F) | (MPa) | (bara) | (psia) | (mW/m K) | (kcal(IT)/(h m K)) | (Btu(IT)/(h ft °F)) | (Btu(IT) in/(h ft2 °F)) | |
| Liquid | 100 | -173 | -280 | 0.1 | 1 | 14.5 | 199.7 | 0.1717 | 0.1154 | 1.385 |
| 111.5 | -161.6 | -259.0 | 0.1 | 1 | 14.5 | 184.1 | 0.1583 | 0.1064 | 1.276 | |
| Gas | 111.5 | -161.6 | -259.0 | 0.1 | 1 | 14.5 | 11.43 | 0.009828 | 0.006604 | 0.07925 |
| 140 | -133 | -208 | 0.1 | 1 | 14.5 | 14.65 | 0.01260 | 0.008465 | 0.1016 | |
| 180 | -93.2 | -136 | 0.1 | 1 | 14.5 | 19.32 | 0.01661 | 0.01116 | 0.1340 | |
| 200 | -73.2 | -99.7 | 0.1 | 1 | 14.5 | 21.94 | 0.01887 | 0.01268 | 0.1521 | |
| 220 | -53.2 | -63.7 | 0.1 | 1 | 14.5 | 23.99 | 0.02063 | 0.01386 | 0.1663 | |
| 240 | -33.2 | -27.7 | 0.1 | 1 | 14.5 | 26.39 | 0.02269 | 0.01525 | 0.1830 | |
| 260 | -13.2 | 8.3 | 0.1 | 1 | 14.5 | 28.88 | 0.02483 | 0.01669 | 0.2002 | |
| 280 | 6.9 | 44.3 | 0.1 | 1 | 14.5 | 31.47 | 0.02706 | 0.01818 | 0.2182 | |
| 300 | 26.9 | 80.3 | 0.1 | 1 | 14.5 | 34.19 | 0.02940 | 0.01975 | 0.2371 | |
| 320 | 46.9 | 116 | 0.1 | 1 | 14.5 | 37.04 | 0.03185 | 0.02140 | 0.2568 | |
| 340 | 66.9 | 152 | 0.1 | 1 | 14.5 | 40.03 | 0.03442 | 0.02313 | 0.2775 | |
| 360 | 86.9 | 188 | 0.1 | 1 | 14.5 | 43.15 | 0.03710 | 0.02493 | 0.2992 | |
| 400 | 127 | 260 | 0.1 | 1 | 14.5 | 49.80 | 0.04282 | 0.02877 | 0.3453 | |
| 500 | 227 | 440 | 0.1 | 1 | 14.5 | 68.34 | 0.05876 | 0.03949 | 0.4738 | |
| 600 | 327 | 620 | 0.1 | 1 | 14.5 | 88.80 | 0.07635 | 0.05131 | 0.6157 | |
| 700 | 427 | 800 | 0.1 | 1 | 14.5 | 110.4 | 0.0949 | 0.06379 | 0.7655 | |
| 800 | 527 | 980 | 0.1 | 1 | 14.5 | 132.5 | 0.1139 | 0.07656 | 0.9187 | |
| 900 | 627 | 1160 | 0.1 | 1 | 14.5 | 154.7 | 0.1330 | 0.08938 | 1.073 | |
| 1000 | 727 | 1340 | 0.1 | 1 | 14.5 | 176.7 | 0.1519 | 0.10210 | 1.225 | |
| Liquid | 100 | -173 | -280 | 1 | 10 | 145 | 200.6 | 0.1725 | 0.1159 | 1.391 |
| 149.1 | -124.0 | -191.2 | 1 | 10 | 145 | 130.7 | 0.1123 | 0.07549 | 0.9059 | |
| Gas | 149.1 | -124.0 | -191.2 | 1 | 10 | 145 | 18.17 | 0.01562 | 0.01050 | 0.1260 |
| 160 | -113 | -172 | 1 | 10 | 145 | 18.79 | 0.01616 | 0.01086 | 0.1303 | |
| 180 | -93.2 | -136 | 1 | 10 | 145 | 20.65 | 0.01776 | 0.01193 | 0.1432 | |
| 200 | -73.2 | -99.7 | 1 | 10 | 145 | 22.74 | 0.01955 | 0.01314 | 0.1577 | |
| 220 | -53.2 | -63.7 | 1 | 10 | 145 | 24.90 | 0.02141 | 0.01439 | 0.1726 | |
| 240 | -33.2 | -27.7 | 1 | 10 | 145 | 27.19 | 0.02338 | 0.01571 | 0.1885 | |
| 260 | -13.2 | 8.3 | 1 | 10 | 145 | 29.60 | 0.02545 | 0.01710 | 0.2052 | |
| 280 | 6.9 | 44.3 | 1 | 10 | 145 | 32.12 | 0.02762 | 0.01856 | 0.2227 | |
| 300 | 26.9 | 80.3 | 1 | 10 | 145 | 34.79 | 0.02991 | 0.02010 | 0.2412 | |
| 320 | 46.9 | 116 | 1 | 10 | 145 | 37.59 | 0.03232 | 0.02172 | 0.2606 | |
| 340 | 66.9 | 152 | 1 | 10 | 145 | 40.54 | 0.03486 | 0.02342 | 0.2811 | |
| 360 | 86.9 | 188 | 1 | 10 | 145 | 43.64 | 0.03752 | 0.02521 | 0.3026 | |
| 400 | 127 | 260 | 1 | 10 | 145 | 50.23 | 0.04319 | 0.02902 | 0.3483 | |
| 500 | 227 | 440 | 1 | 10 | 145 | 68.68 | 0.05905 | 0.03968 | 0.4762 | |
| 600 | 327 | 620 | 1 | 10 | 145 | 89.08 | 0.07660 | 0.05147 | 0.6176 | |
| Liquid | 100 | -173 | -280 | 5 | 50 | 725 | 204.5 | 0.1758 | 0.1181 | 1.418 |
| Supercritical phase |
200 | -73.2 | -99.7 | 5 | 50 | 725 | 40.61 | 0.03492 | 0.02347 | 0.2816 |
| 300 | 26.9 | 80.3 | 5 | 50 | 725 | 38.48 | 0.03309 | 0.02223 | 0.2668 | |
| 400 | 127 | 260 | 5 | 50 | 725 | 52.69 | 0.04531 | 0.03045 | 0.3653 | |
| 500 | 227 | 440 | 5 | 50 | 725 | 70.51 | 0.06063 | 0.04074 | 0.4889 | |
| 600 | 327 | 620 | 5 | 50 | 725 | 90.50 | 0.07781 | 0.05229 | 0.6275 | |
| Liquid | 100 | -173 | -280 | 10 | 100 | 1450 | 209.1 | 0.1798 | 0.1208 | 1.450 |
| Supercritical phase |
200 | -73.2 | -100 | 10 | 100 | 1450 | 84.23 | 0.07243 | 0.04867 | 0.5840 |
| 240 | -33.2 | -27.7 | 10 | 100 | 1450 | 49.74 | 0.04277 | 0.02874 | 0.3449 | |
| 260 | -13.2 | 8.3 | 10 | 100 | 1450 | 44.85 | 0.03856 | 0.02591 | 0.3110 | |
| 280 | 6.9 | 44.3 | 10 | 100 | 1450 | 43.81 | 0.03767 | 0.02531 | 0.3038 | |
| 300 | 26.9 | 80.3 | 10 | 100 | 1450 | 44.37 | 0.03815 | 0.02564 | 0.3076 | |
| 320 | 46.9 | 116 | 10 | 100 | 1450 | 45.79 | 0.03937 | 0.02646 | 0.3175 | |
| 340 | 66.9 | 152 | 10 | 100 | 1450 | 47.75 | 0.04106 | 0.02759 | 0.3311 | |
| 360 | 86.9 | 188 | 10 | 100 | 1450 | 50.09 | 0.04307 | 0.02894 | 0.3473 | |
| 400 | 127 | 260 | 10 | 100 | 1450 | 55.61 | 0.04782 | 0.03213 | 0.3856 | |
| 500 | 227 | 440 | 10 | 100 | 1450 | 72.78 | 0.06258 | 0.04205 | 0.5046 | |
| Supercritical phase |
200 | -73.2 | -100 | 100 | 1000 | 14500 | 188.1 | 0.1617 | 0.1087 | 1.304 |
| 300 | 26.9 | 80.3 | 100 | 1000 | 14500 | 137.7 | 0.1184 | 0.07955 | 0.9546 | |
| 400 | 127 | 260 | 100 | 1000 | 14500 | 120.4 | 0.1035 | 0.06955 | 0.8347 | |
| 500 | 227 | 440 | 100 | 1000 | 14500 | 120.9 | 0.1039 | 0.06984 | 0.8381 | |
| 600 | 327 | 620 | 100 | 1000 | 14500 | 130.4 | 0.1121 | 0.07532 | 0.9039 | |
Thermal conductivity units conversion:
Thermal conductivity unit converter
british thermal unit(international)/(foot hour degree fahrenheit) (Btu(IT)/(ft h°F), british thermal unit(international)/(inch hour degree fahrenheit) (Btu(IT)/(in h°F), british thermal unit(international)*inch/(square foot*hour*degree fahrenheit) ((Btu(IT) in)/(ft² h°F)), kilocalorie/(meter hour degree celcius) (kcal/(m h°C)), joule/(centimeter second degree kelvin) (J/(cm s K)), watt/(meter degree kelvin) (W/(m°C)),
- 1 Btu(IT)/(ft h°F) = 1/12 Btu(IT)/(in h°F) = 0.08333 Btu(IT)/(in h°F) = 12 Btu(IT)in/(ft2h°F) = 1.488 kcal/(m h°C) = 0.01731 J/(cm s K) = 1.731 W/(m K)
- 1 Btu(IT)/(in h°F) = 12 Btu(IT)/(ft h°F) = 144 Btu(IT)in/(ft2h°F) = 17.858 kcal/(m h°C) = 0.20769 J/(cm s K)= 20.769 W/(m K)
- 1 (Btu(IT) in)/(ft² h°F) = 0.08333 Btu(IT)/(ft h°F) = 0.00694 Btu(IT)/(in h°F) = 0.12401 kcal/(m h°C) = 0.001442 J/(cm s K) = 0.1442 W/(m K)
- 1 J/(cm s K) = 100 W/(m K) = 57.789 Btu(IT)/(ft h°F) = 4.8149 Btu(IT)/(in h°F) = 693.35 (Btu(IT) in)/(ft² h°F) = 85.984 kcal/(m h°C)
- 1 kcal/(m h°C) = 0.6720 Btu(IT)/(ft h°F) = 0.05600 Btu(IT)/(in h°F) = 8.0636 (Btu(IT) in)/(ft2h°F) = 0.01163 J/(cm s K) = 1.163 W/(m K)
- 1 W/(m K) = 0.01 J/(cm s K) = 0.5779 Btu(IT)/(ft h°F) = 0.04815 Btu(IT)/(in h°F) = 6.9335 (Btu(IT) in)/(ft² h°F) = 0.85984 kcal/(m h°C)



