Air - Specific Heat vs. Pressure at Constant Temperature
Figures and tables with isobaric (Cp) and isochoric (Cv) specific heat of air at constant temperature and pressure ranging 0.01 to 10000 bara.
Specific heat (C) is the amount of heat required to change the temperature of a mass unit of a substance by one degree.
- Isobaric specific heat (Cp) is used for air in a constant pressure (ΔP = 0) system.
- I sochoric specific heat (Cv) is used for air in a constant-volume , (= isovolumetric or isometric ) closed system.
The specific heat of dry air - CP and CV - will vary with pressure and temperature. This may influence on the accuracy of industrial air handling process calculations. When calculating mass and volume flow of air in heated or cooled systems with high accuracy - the specific heat (= heat capacity) should be corrected according values in the figures and table below.
See also other properties of Air at varying temperature and pressure: Density and specific weight at varying temperature, Density at varying pressure, Diffusion Coefficients for Gases in Air, Prandtl Number, Specific heat at varying temperature, Thermal Conductivity, Thermal Diffusivity, Properties at gas-liquid equilibrium conditions and Air thermophysical properties at standard conditions and Composition and molecular weight,
as well as Specific heat of Ammonia, Butane, Carbon dioxide, Carbon monoxide, Ethane, Ethanol, Ethylene, Hydrogen, Methane, Methanol, Nitrogen, Oxygen, Propane and Water.
Specific Heat of dry air at constant temperature 20 oC / 68°F at various pressures:
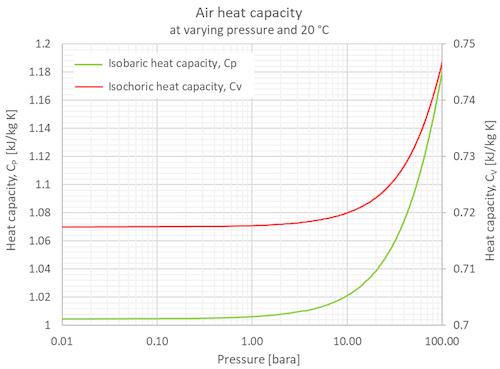
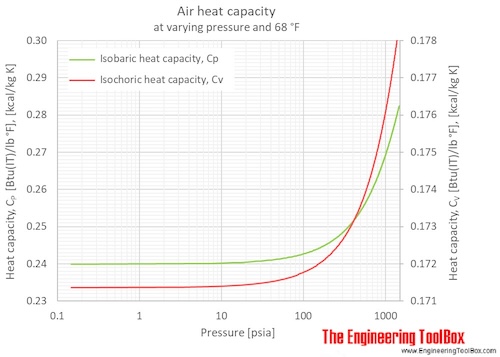
Specific Heat of dry air at selected temperatures at various pressures:
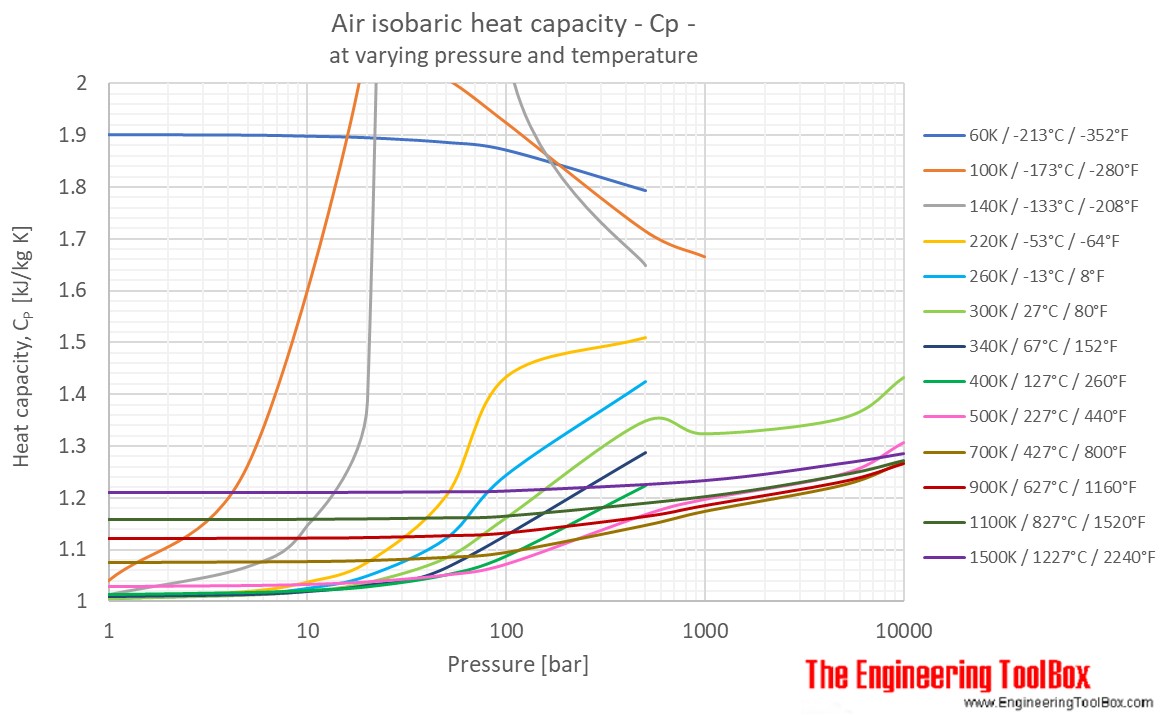
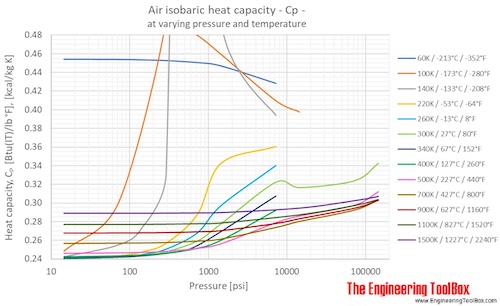
Specific Heat of dry air at constant temperature 20 oC (68°F) at varying pressures:
For full table with Isochloric Heat Capacity - rotate the screen!
| Pressure | Isobaric Specific Heat, Cp | Isochoric Specific Heat, Cv | Specific Heat Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (atm) | (psia) | (kPa) | (kJ/kgK) | (kWh/(kg K)) | (kcal/(kg °C)), (Btu/(lb °F)) | (kcal/(lb °F)) | (kJ/kgK) | (kWh/(kg K)) | (kcal/(kg °C)), (Btu/(lb °F)) | (kcal/(lb °F)) | Cp/Cv |
| 0.01 | 0.147 | 1.01325 | 1.0045 | 0.00027903 | 0.2399 | 0.1959 | 0.71740 | 0.00019928 | 0.1713 | 0.1399 | 1.4002 |
| 0.1 | 1.47 | 10.1325 | 1.0049 | 0.00027914 | 0.2400 | 0.1960 | 0.71763 | 0.00019934 | 0.1714 | 0.1399 | 1.4003 |
| 0.4 | 5.88 | 40.53 | 1.0053 | 0.00027925 | 0.2401 | 0.1960 | 0.71766 | 0.00019935 | 0.1714 | 0.1400 | 1.4008 |
| 11) | 14.7 | 101.325 | 1.0061 | 0.00027947 | 0.2403 | 0.1962 | 0.71767 | 0.00019935 | 0.1714 | 0.1400 | 1.4019 |
| 7 | 102.9 | 709.28 | 1.0162 | 0.00028228 | 0.2427 | 0.1982 | 0.71913 | 0.00019976 | 0.1718 | 0.1402 | 1.4131 |
| 10 | 147 | 1013.25 | 1.0216 | 0.00028378 | 0.2440 | 0.1992 | 0.72005 | 0.00020001 | 0.1720 | 0.1404 | 1.4188 |
| 40 | 588 | 4053 | 1.0756 | 0.00029878 | 0.2569 | 0.2098 | 0.72858 | 0.00020238 | 0.1740 | 0.1421 | 1.4763 |
| 70 | 1029 | 7092.8 | 1.1305 | 0.00031403 | 0.2700 | 0.2205 | 0.73778 | 0.00020494 | 0.1762 | 0.1439 | 1.5323 |
| 100 | 1470 | 10132.5 | 1.1824 | 0.00032845 | 0.2824 | 0.2306 | 0.74703 | 0.00020751 | 0.1784 | 0.1457 | 1.5828 |
Unit conversion:
british thermal unit(International table) = (Btu(IT)), degree celcius = (°C), degree fahrenheit = (°F), degree kelvin = (K), degree rankin = (°R), joule = (J), kilocalorie(International table) = (kcal(IT)), kilogram = (kg), kilojoule = (kJ), kilowatthour = (kWh), mole = (mol), pound =(lb)
K in the units can be replaced by °C, and vise versa. °R in the units can be replaced by °F, and vise versa.
- 1 Btu/(lb °F) = 1 Btu/(lb °R) = 1 kcal(IT)/(kg °C) = 1 kcal(IT)/(kg K) = 4186.8 J/(kg K) = 0.81647 kcal(IT)/(lb °F) = 1.163×10-3 kWh/(kg K)
- 1 J/(kg K) = 1 J/(kg °C) = 2.3885×10-4 kcal(IT)/(kg oC) = 2.3885×10-4 Btu/(lb °F) = 1.9501×10-4 kcal(IT)/(lb °F)
- 1 kcal(IT)/(kg °C) = 1 Btu/(lb °F) = 4186.8 J/(kg K) = 0.81647 kcal(IT)/(lb °F) = 1.163×10-3 kWh/(kg K)
- 1 kcal(IT)/(lb °F) = 1.2248 Btu/(lb °F) = 1.2248 kcal(IT)/(kg °C) = 5127.9 J/(kg K)
- 1 kJ/kgK = 1 kJ/(kg °C) = 1000 J/(kg K) = 1000 J/(kg °C) = 0.23885 kcal(IT)/(kg °C) = 0.23885 Btu/(lb °F) = 0.19501 kcal(IT)/(lb °F) = 2.7778×10-4 kWh/(kg K)
- 1 kWh/(kg K) = 0.85985 kcal(IT)/(kg °C) = 0.85985 Btu/(lb °F) = 3.6 kJ/kgK
- 1 mol of air = 28.96546 g



