Methanol - Specific Heat vs. Temperature and Pressure
Online calculator, figures and tables showing isobaric and isochoric specific heat of methanol, CH3OH, at varying temperature - Imperial and SI Units.
Specific heat (C) is the amount of heat required to change the temperature of a mass unit of a substance by one degree.
- Isobaric specific heat (Cp) is used for a substance in a constant pressure (ΔP = 0) system.
- I sochoric specific heat (Cv) is used for a substance in a constant-volume , (= isovolumetric or isometric ) closed system.
At ambient pressure and temperature the isobaric specific heat, CP, of liquid methanol is 2.53 (kJ/kg K) or 0.605 (Btu/lb °F) = (cal/g K), while the isochoric specific heat, CV, is 2.12 (kJ/kg K) or 0.506 (Btu/lb °F) = (cal/g K).
However, the specific heat - CP and CV - will vary with temperature. This may influence on the accuracy of methanol conditioning and industrial methanol handling process calculations. When calculating mass and volume flow of methanol in heated or cooled systems with high accuracy - the specific heat should be corrected according values in the figures and tables below, or found by use of the calculator.
- Specific heat unit conversion
Online Liquid Methanol Specific Heat Calculator
The calculator below can be used to estimate the specific heat of liquid methanol at constant volum(CV) or constant pressure(CP) and at given temperature. For practical purpose the specific heat of liquid methanol is constant with varying pressure up to critical point (240°C, 82.16 bara / 464°F, 1192 psia).
Note that the boiling point of methanol at atmospheric pressure is 64.7°C (148.5°F), and methanol must therefor be pressurized to be present as liquid at higher temperatures.
The output heat capacity is given as kJ/(mol*K), kJ/(kg*K), kWh/(kg*K), kcal/(kg*K), Btu(IT)/(mol*°R) and Btu(IT)/(lbm *°R)
See also Methanol at varying temperature and pressure: Density and specific weight and Dynamic and kinematic viscosity, and Thermophysical properties at standard conditions,
as well as Specific heat of Air - at Constant Pressure and Varying Temperature, Air - at Constant Temperature and Varying Pressure, Ammonia, Butane, Carbon dioxide, Carbon monoxide, Ethane, Ethanol, Ethylene, Hydrogen, Methane, Nitrogen, Oxygen, Propane and Water .
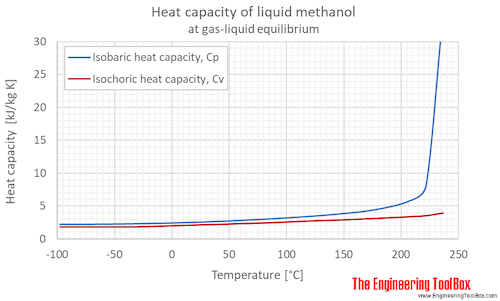
Isobaric, CP, and isochoric, CV, specific heat of methanol at gas-liquid equilibrium pressure, SI and Imperial units:
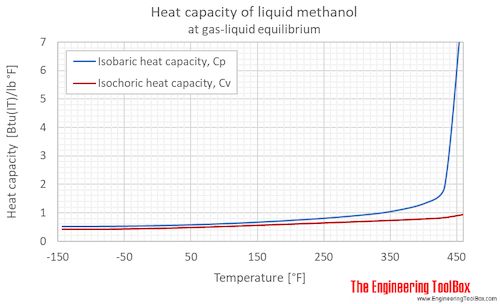
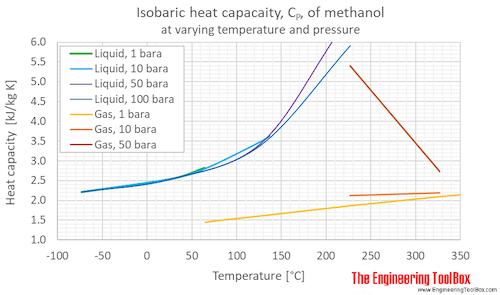
Isobaric specific heat, CP, of methanol at varying temperature and pressure, SI and Imperial units:
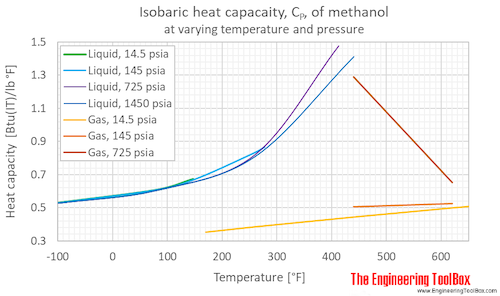
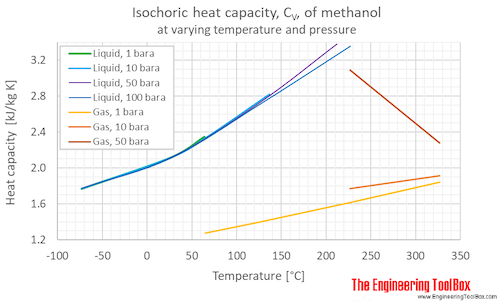
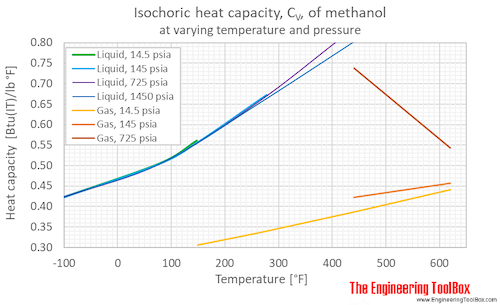
Isochoric specific heat, CV, of methanol at varying temperature and pressure, SI and Imperial units:
Isobaric specific heat, CP, of methanol at given temperatures and pressures:
For full table with Isobaric Specific Heat Units - rotate the screen!
| State | Temperature | Pressure | Isobaric Specific Heat, CP | Cp/Cv | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (K) | (°C) | (°F) | (MPa) | (bara) | (psia) | (kJ/mol K) | (kJ/kg K) | (Wh/(kg K)) | (kcal(IT)/(kg K)), (Btu(IT)/lb °F) | (Btu(IT)/(mol °F)) | (-) | |
|
Liquid at equilibrium |
175.61 | -97.54 | -143.57 | 1.86E-07 | 1.86E-06 | 2.70E-05 | 0.07039 | 2.197 | 0.6102 | 0.5247 | 0.03706 | 1.24 |
| 210 | -63.2 | -81.7 | 1.98E-05 | 1.98E-04 | 2.88E-03 | 0.07122 | 2.223 | 0.6174 | 0.5308 | 0.03750 | 1.25 | |
| 240 | -33.2 | -27.7 | 3.63E-04 | 3.63E-03 | 0.0527 | 0.07314 | 2.283 | 0.6341 | 0.5452 | 0.03851 | 1.23 | |
| 270 | -3.1 | 26.3 | 3.32E-03 | 0.0332 | 0.481 | 0.07648 | 2.387 | 0.6630 | 0.5701 | 0.04027 | 1.22 | |
| 298 | 25.0 | 77.0 | 0.0166 | 0.166 | 2.41 | 0.08120 | 2.534 | 0.7039 | 0.6053 | 0.04276 | 1.20 | |
| 300 | 26.9 | 80.3 | 0.0187 | 0.187 | 2.71 | 0.08158 | 2.546 | 0.7073 | 0.6081 | 0.04296 | 1.20 | |
| 330 | 56.9 | 134 | 0.0745 | 0.745 | 10.8 | 0.08851 | 2.762 | 0.7673 | 0.6597 | 0.04660 | 1.20 | |
| 360 | 86.9 | 188 | 0.230 | 2.30 | 33.3 | 0.09716 | 3.032 | 0.8423 | 0.7243 | 0.05116 | 1.22 | |
| 390 | 117 | 242 | 0.586 | 5.86 | 85.0 | 0.1078 | 3.363 | 0.9342 | 0.8033 | 0.05674 | 1.25 | |
| 420 | 147 | 296 | 1.29 | 12.9 | 187 | 0.1213 | 3.787 | 1.052 | 0.9044 | 0.06389 | 1.31 | |
| 450 | 177 | 350 | 2.54 | 25.4 | 369 | 0.1412 | 4.407 | 1.224 | 1.053 | 0.07435 | 1.43 | |
| 480 | 207 | 404 | 4.57 | 45.7 | 663 | 0.1835 | 5.725 | 1.590 | 1.367 | 0.09660 | 1.72 | |
| 495 | 97 | 207 | 5.98 | 59.8 | 867 | 0.2572 | 8.026 | 2.229 | 1.917 | 0.1354 | 2.29 | |
| 510 | 237 | 458 | 7.75 | 77.5 | 1124 | 1.109 | 34.60 | 9.612 | 8.265 | 0.5838 | 8.76 | |
|
Gas at equilibrium |
175.61 | -97.54 | -143.57 | 1.86E-07 | 1.86E-06 | 2.70E-05 | 0.04029 | 1.257 | 0.3493 | 0.3003 | 0.02121 | 1.26 |
| 210 | -63.2 | -81.7 | 1.98E-05 | 1.98E-04 | 2.88E-03 | 0.04939 | 1.541 | 0.4282 | 0.3682 | 0.02601 | 1.23 | |
| 240 | -33.2 | -27.7 | 3.63E-04 | 3.63E-03 | 0.0527 | 0.06797 | 2.121 | 0.5893 | 0.5067 | 0.03579 | 1.21 | |
| 270 | -3.1 | 26.3 | 3.32E-03 | 0.0332 | 0.481 | 0.09300 | 2.902 | 0.8062 | 0.6932 | 0.04897 | 1.21 | |
| 298 | 25.0 | 77.0 | 0.0166 | 0.166 | 2.41 | 0.1160 | 3.620 | 1.006 | 0.8647 | 0.06108 | 1.23 | |
| 300 | 26.9 | 80.3 | 0.0187 | 0.187 | 2.71 | 0.1174 | 3.664 | 1.018 | 0.8751 | 0.06182 | 1.23 | |
| 330 | 56.9 | 134 | 0.0745 | 0.745 | 10.8 | 0.1375 | 4.291 | 1.192 | 1.025 | 0.07240 | 1.27 | |
| 360 | 86.9 | 188 | 0.230 | 2.30 | 33.3 | 0.1556 | 4.856 | 1.349 | 1.160 | 0.08193 | 1.33 | |
| 390 | 117 | 242 | 0.586 | 5.86 | 85.0 | 0.1792 | 5.592 | 1.553 | 1.336 | 0.09434 | 1.43 | |
| 420 | 147 | 296 | 1.29 | 12.9 | 187 | 0.2199 | 6.862 | 1.906 | 1.639 | 0.1158 | 1.62 | |
| 450 | 177 | 350 | 2.54 | 25.4 | 369 | 0.2650 | 8.271 | 2.298 | 1.975 | 0.1395 | 1.86 | |
| 480 | 207 | 404 | 4.57 | 45.7 | 663 | 0.2796 | 8.726 | 2.424 | 2.084 | 0.1472 | 2.22 | |
| 495 | 222 | 431 | 5.98 | 59.8 | 867 | 0.4245 | 13.25 | 3.680 | 3.164 | 0.2235 | 3.37 | |
| 510 | 237 | 458 | 7.75 | 77.5 | 1124 | 1.910 | 59.60 | 16.55 | 14.23 | 1.006 | 14.4 | |
| Liquid | 200 | -73.2 | -99.7 | 0.1 | 1 | 14.5 | 0.07094 | 2.214 | 0.6150 | 0.5288 | 0.03736 | 1.25 |
| 300 | 26.9 | 80.3 | 0.1 | 1 | 14.5 | 0.08158 | 2.546 | 0.7072 | 0.6081 | 0.04296 | 1.20 | |
| 337.3 | 64.15 | 147.47 | 0.1 | 1 | 14.5 | 0.09045 | 2.823 | 0.7841 | 0.6742 | 0.04763 | 1.20 | |
| Gas | 337.3 | 64.15 | 147.47 | 0.1 | 1 | 14.5 | 0.1419 | 4.428 | 1.230 | 1.058 | 0.07470 | 1.28 |
| 400 | 127 | 260 | 0.1 | 1 | 14.5 | 0.05421 | 1.692 | 0.4699 | 0.4041 | 0.02854 | 1.21 | |
| 500 | 227 | 440 | 0.1 | 1 | 14.5 | 0.06038 | 1.884 | 0.5234 | 0.4501 | 0.03179 | 1.17 | |
| 600 | 327 | 620 | 0.1 | 1 | 14.5 | 0.06744 | 2.105 | 0.5847 | 0.5027 | 0.03551 | 1.14 | |
| Liquid | 200 | -73.2 | -99.7 | 1 | 10 | 145 | 0.07093 | 2.214 | 0.6149 | 0.5287 | 0.03735 | 1.25 |
| 300 | 26.9 | 80.3 | 1 | 10 | 145 | 0.08154 | 2.545 | 0.7069 | 0.6078 | 0.04294 | 1.20 | |
| 400 | 127 | 260 | 1 | 10 | 145 | 0.1118 | 3.488 | 0.9690 | 0.8331 | 0.05885 | 1.27 | |
| 409.75 | 136.60 | 277.88 | 1 | 10 | 145 | 0.1162 | 3.627 | 1.008 | 0.8664 | 0.06120 | 1.29 | |
| Gas | 409.75 | 136.60 | 277.88 | 1 | 10 | 145 | 0.2033 | 6.346 | 1.763 | 1.516 | 0.1071 | 1.54 |
| 500 | 227 | 440 | 1 | 10 | 145 | 0.06807 | 2.124 | 0.5901 | 0.5074 | 0.03584 | 1.20 | |
| 600 | 327 | 620 | 1 | 10 | 145 | 0.07037 | 2.196 | 0.6100 | 0.5245 | 0.03705 | 1.15 | |
| Liquid | 200 | -73.2 | -99.7 | 5 | 50 | 725 | 0.07088 | 2.212 | 0.6145 | 0.5284 | 0.03732 | 1.25 |
| 300 | 26.9 | 80.3 | 5 | 50 | 725 | 0.08138 | 2.540 | 0.7055 | 0.6066 | 0.04285 | 1.20 | |
| 400 | 127 | 260 | 5 | 50 | 725 | 0.1103 | 3.442 | 0.9561 | 0.8221 | 0.05807 | 1.26 | |
| 484.95 | 211.80 | 413.24 | 5 | 50 | 725 | 0.1984 | 6.191 | 1.720 | 1.479 | 0.1044 | 1.83 | |
| Gas | 484.95 | 211.80 | 413.24 | 5 | 50 | 725 | 0.3226 | 10.07 | 2.797 | 2.405 | 0.1699 | 2.58 |
| 500 | 227 | 440 | 5 | 50 | 725 | 0.1732 | 5.404 | 1.501 | 1.291 | 0.09117 | 1.75 | |
| 600 | 327 | 620 | 5 | 50 | 725 | 0.08749 | 2.730 | 0.7585 | 0.6522 | 0.04607 | 1.20 | |
| Liquid | 200 | -73.2 | -99.7 | 10 | 100 | 1450 | 0.07082 | 2.210 | 0.6140 | 0.5279 | 0.03729 | 1.24 |
| 300 | 26.9 | 80.3 | 10 | 100 | 1450 | 0.08120 | 2.534 | 0.7039 | 0.6052 | 0.04275 | 1.20 | |
| 400 | 127 | 260 | 10 | 100 | 1450 | 0.1088 | 3.396 | 0.9432 | 0.8110 | 0.05729 | 1.25 | |
| 500 | 227 | 440 | 10 | 100 | 1450 | 0.1896 | 5.917 | 1.644 | 1.413 | 0.09983 | 1.76 | |
| Supercritical phase |
600 | 327 | 620 | 10 | 100 | 1450 | 0.1212 | 3.783 | 1.051 | 0.9036 | 0.06383 | 1.36 |
| Liquid | 200 | -73.2 | -99.7 | 100 | 1000 | 14504 | 0.06899 | 2.153 | 0.5981 | 0.5143 | 0.03633 | 1.19 |
| 300 | 26.9 | 80.3 | 100 | 1000 | 14504 | 0.07982 | 2.491 | 0.6920 | 0.5950 | 0.04203 | 1.18 | |
| 400 | 127 | 260 | 100 | 1000 | 14504 | 0.09895 | 3.088 | 0.8578 | 0.7376 | 0.05210 | 1.19 | |
| 500 | 227 | 440 | 100 | 1000 | 14504 | 0.12152 | 3.793 | 1.053 | 0.9058 | 0.06399 | 1.27 | |
| Supercritical phase |
600 | 327 | 620 | 100 | 1000 | 14504 | 0.13787 | 4.303 | 1.195 | 1.028 | 0.07260 | 1.32 |
Isochoric specific heat, CV, of methanol at given temperatures and pressures:
For full table with Isochloric Specific Heat Units - rotate the screen!
| State | Temperature | Pressure | Isochoric Specific Heat, CV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (K) | (°C) | (°F) | (MPa) | (bara) | (psia) | (kJ/mol K) | (kJ/kg K) | (Wh/(kg K)) | (kcal(IT)/(kg K)), (Btu(IT)/lb °F) | (Btu(IT)/(mol °F)) | |
|
Liquid at equilibrium |
175.61 | -97.54 | -143.57 | 1.86E-07 | 1.86E-06 | 2.70E-05 | 0.05673 | 1.770 | 0.4918 | 0.4229 | 0.02987 |
| 210 | -63.2 | -81.7 | 1.98E-05 | 1.98E-04 | 2.88E-03 | 0.05707 | 1.781 | 0.4948 | 0.4254 | 0.03005 | |
| 240 | -33.2 | -27.7 | 3.63E-04 | 3.63E-03 | 0.0527 | 0.05928 | 1.850 | 0.5139 | 0.4418 | 0.03121 | |
| 270 | -3.1 | 26.3 | 3.32E-03 | 0.0332 | 0.481 | 0.06292 | 1.964 | 0.5454 | 0.4690 | 0.03313 | |
| 298 | 25.0 | 77.0 | 0.0166 | 0.166 | 2.41 | 0.06750 | 2.107 | 0.5852 | 0.5032 | 0.03554 | |
| 300 | 26.9 | 80.3 | 0.0187 | 0.187 | 2.71 | 0.06786 | 2.118 | 0.5883 | 0.5059 | 0.03573 | |
| 330 | 56.9 | 134 | 0.0745 | 0.745 | 10.8 | 0.07367 | 2.299 | 0.6387 | 0.5492 | 0.03879 | |
| 360 | 86.9 | 188 | 0.230 | 2.30 | 33.3 | 0.07988 | 2.493 | 0.6925 | 0.5954 | 0.04206 | |
| 390 | 117 | 242 | 0.586 | 5.86 | 85.0 | 0.08619 | 2.690 | 0.7472 | 0.6425 | 0.04538 | |
| 420 | 147 | 296 | 1.29 | 12.9 | 187 | 0.09251 | 2.887 | 0.8020 | 0.6896 | 0.04871 | |
| 450 | 177 | 350 | 2.54 | 25.4 | 369 | 0.09903 | 3.091 | 0.8585 | 0.7382 | 0.05215 | |
| 480 | 207 | 404 | 4.57 | 45.7 | 663 | 0.1067 | 3.329 | 0.9247 | 0.7951 | 0.05616 | |
| 495 | 97 | 207 | 5.98 | 59.8 | 867 | 0.1125 | 3.511 | 0.9753 | 0.8386 | 0.05924 | |
| 510 | 237 | 458 | 7.75 | 77.5 | 1124 | 0.1265 | 3.949 | 1.097 | 0.9432 | 0.06663 | |
|
Gas at equilibrium |
175.61 | -97.54 | -143.57 | 1.86E-07 | 1.86E-06 | 2.70E-05 | 0.03187 | 0.9948 | 0.2763 | 0.2376 | 0.01678 |
| 210 | -63.2 | -81.7 | 1.98E-05 | 1.98E-04 | 2.88E-03 | 0.04010 | 1.252 | 0.3477 | 0.2989 | 0.02112 | |
| 240 | -33.2 | -27.7 | 3.63E-04 | 3.63E-03 | 0.0527 | 0.05632 | 1.758 | 0.4883 | 0.4198 | 0.02966 | |
| 270 | -3.1 | 26.3 | 3.32E-03 | 0.0332 | 0.481 | 0.07706 | 2.405 | 0.6680 | 0.5744 | 0.04057 | |
| 298 | 25.0 | 77.0 | 0.0166 | 0.166 | 2.41 | 0.09450 | 2.949 | 0.8192 | 0.7044 | 0.04976 | |
| 300 | 26.9 | 80.3 | 0.0187 | 0.187 | 2.71 | 0.09558 | 2.983 | 0.8286 | 0.7125 | 0.05033 | |
| 330 | 56.9 | 134 | 0.0745 | 0.745 | 10.8 | 0.1086 | 3.389 | 0.9415 | 0.8095 | 0.05718 | |
| 360 | 86.9 | 188 | 0.230 | 2.30 | 33.3 | 0.1174 | 3.663 | 1.017 | 0.8748 | 0.06180 | |
| 390 | 117 | 242 | 0.586 | 5.86 | 85.0 | 0.1255 | 3.915 | 1.088 | 0.9352 | 0.06606 | |
| 420 | 147 | 296 | 1.29 | 12.9 | 187 | 0.1359 | 4.240 | 1.178 | 1.013 | 0.07154 | |
| 450 | 177 | 350 | 2.54 | 25.4 | 369 | 0.1424 | 4.444 | 1.234 | 1.061 | 0.07497 | |
| 480 | 207 | 404 | 4.57 | 45.7 | 663 | 0.1262 | 3.938 | 1.094 | 0.9406 | 0.06644 | |
| 495 | 222 | 431 | 5.98 | 59.8 | 867 | 0.1261 | 3.935 | 1.093 | 0.9398 | 0.06639 | |
| 510 | 237 | 458 | 7.75 | 77.5 | 1124 | 0.1326 | 4.138 | 1.149 | 0.9883 | 0.06982 | |
| Liquid | 200 | -73.2 | -99.7 | 0.1 | 1 | 14.5 | 0.05670 | 1.770 | 0.4916 | 0.4227 | 0.02986 |
| 300 | 26.9 | 80.3 | 0.1 | 1 | 14.5 | 0.06786 | 2.118 | 0.5883 | 0.5059 | 0.03573 | |
| 337.3 | 64.15 | 147.47 | 0.1 | 1 | 14.5 | 0.07516 | 2.346 | 0.6516 | 0.5603 | 0.03958 | |
| Gas | 337.3 | 64.15 | 147.47 | 0.1 | 1 | 14.5 | 0.1110 | 3.464 | 0.9623 | 0.8274 | 0.05845 |
| 400 | 127 | 260 | 0.1 | 1 | 14.5 | 0.04497 | 1.404 | 0.3899 | 0.3352 | 0.02368 | |
| 500 | 227 | 440 | 0.1 | 1 | 14.5 | 0.05182 | 1.617 | 0.4493 | 0.3863 | 0.02729 | |
| 600 | 327 | 620 | 0.1 | 1 | 14.5 | 0.05907 | 1.843 | 0.5120 | 0.4403 | 0.03110 | |
| Liquid | 200 | -73.2 | -99.7 | 1 | 10 | 145 | 0.05672 | 1.770 | 0.4918 | 0.4228 | 0.02987 |
| 300 | 26.9 | 80.3 | 1 | 10 | 145 | 0.06785 | 2.117 | 0.5882 | 0.5057 | 0.03573 | |
| 400 | 127 | 260 | 1 | 10 | 145 | 0.08826 | 2.754 | 0.7651 | 0.6579 | 0.04647 | |
| 409.75 | 136.60 | 277.88 | 1 | 10 | 145 | 0.09035 | 2.820 | 0.7832 | 0.6735 | 0.04757 | |
| Gas | 409.75 | 136.60 | 277.88 | 1 | 10 | 145 | 0.1320 | 4.121 | 1.145 | 0.9842 | 0.06952 |
| 500 | 227 | 440 | 1 | 10 | 145 | 0.05668 | 1.769 | 0.4913 | 0.4225 | 0.02984 | |
| 600 | 327 | 620 | 1 | 10 | 145 | 0.06134 | 1.914 | 0.5318 | 0.4573 | 0.03230 | |
| Liquid | 200 | -73.2 | -99.7 | 5 | 50 | 725 | 0.05682 | 1.773 | 0.4926 | 0.4235 | 0.02992 |
| 300 | 26.9 | 80.3 | 5 | 50 | 725 | 0.06780 | 2.116 | 0.5877 | 0.5054 | 0.03570 | |
| 400 | 127 | 260 | 5 | 50 | 725 | 0.08768 | 2.736 | 0.7601 | 0.6535 | 0.04617 | |
| 484.95 | 211.80 | 413.24 | 5 | 50 | 725 | 0.1083 | 3.379 | 0.9385 | 0.8070 | 0.05701 | |
| Gas | 484.95 | 211.80 | 413.24 | 5 | 50 | 725 | 0.1250 | 3.901 | 1.084 | 0.9317 | 0.06581 |
| 500 | 227 | 440 | 5 | 50 | 725 | 0.09898 | 3.089 | 0.8580 | 0.7378 | 0.05212 | |
| 600 | 327 | 620 | 5 | 50 | 725 | 0.07293 | 2.276 | 0.6322 | 0.5436 | 0.03840 | |
| Liquid | 200 | -73.2 | -99.7 | 10 | 100 | 1450 | 0.05694 | 1.777 | 0.4936 | 0.4244 | 0.02998 |
| 300 | 26.9 | 80.3 | 10 | 100 | 1450 | 0.06775 | 2.114 | 0.5873 | 0.5050 | 0.03567 | |
| 400 | 127 | 260 | 10 | 100 | 1450 | 0.08709 | 2.718 | 0.7550 | 0.6492 | 0.04586 | |
| 500 | 227 | 440 | 10 | 100 | 1450 | 0.1076 | 3.358 | 0.9328 | 0.8021 | 0.05666 | |
| Supercritical phase |
600 | 327 | 620 | 10 | 100 | 1450 | 0.08887 | 2.773 | 0.7704 | 0.6624 | 0.04679 |
| Liquid | 200 | -73.2 | -99.7 | 100 | 1000 | 14504 | 0.05783 | 1.805 | 0.5013 | 0.4310 | 0.03045 |
| 300 | 26.9 | 80.3 | 100 | 1000 | 14504 | 0.06789 | 2.119 | 0.5885 | 0.5061 | 0.03575 | |
| 400 | 127 | 260 | 100 | 1000 | 14504 | 0.08282 | 2.585 | 0.7180 | 0.6174 | 0.04361 | |
| 500 | 227 | 440 | 100 | 1000 | 14504 | 0.09569 | 2.987 | 0.8296 | 0.7133 | 0.05039 | |
| Supercritical phase |
600 | 327 | 620 | 100 | 1000 | 14504 | 0.1041 | 3.248 | 0.9021 | 0.7757 | 0.05479 |
british thermal unit(International table) = (Btu(IT)), degree celcius = (°C), degree fahrenheit = (°F), degree kelvin = (K), degree rankin = (°R), joule = (J), kilocalorie(International table) = (kcal(IT)), kilogram = (kg), kilojoule = (kJ), kilowatthour = (kWh), mole = (mol), pound =(lb)
K in the units can be replaced by °C, and vise versa. °R in the units can be replaced by °F, and vise versa.
- 1 Btu/(lb °F) = 1 Btu/(lb °R) = 1 kcal(IT)/(kg °C) = 1 kcal(IT)/(kg K) = 4186.8 J/(kg K) = 0.81647 kcal(IT)/(lb °F) = 1.163×10-3 kWh/(kg K)
- 1 J/(kg K) = 1 J/(kg °C) = 2.3885×10-4 kcal(IT)/(kg oC) = 2.3885×10-4 Btu/(lb °F) = 1.9501×10-4 kcal(IT)/(lb °F)
- 1 kcal(IT)/(kg °C) = 1 Btu/(lb °F) = 4186.8 J/(kg K) = 0.81647 kcal(IT)/(lb °F) = 1.163×10-3 kWh/(kg K)
- 1 kcal(IT)/(lb °F) = 1.2248 Btu/(lb °F) = 1.2248 kcal(IT)/(kg °C) = 5127.9 J/(kg K)
- 1 kJ/kgK = 1 kJ/(kg °C) = 1000 J/(kg K) = 1000 J/(kg °C) = 0.23885 kcal(IT)/(kg °C) = 0.23885 Btu/(lb °F) = 0.19501 kcal(IT)/(lb °F) = 2.7778×10-4 kWh/(kg K)
- 1 kWh/(kg K) = 0.85985 kcal(IT)/(kg °C) = 0.85985 Btu/(lb °F) = 3.6 kJ/kgK
- 1 mol of air = 28.96546 g



