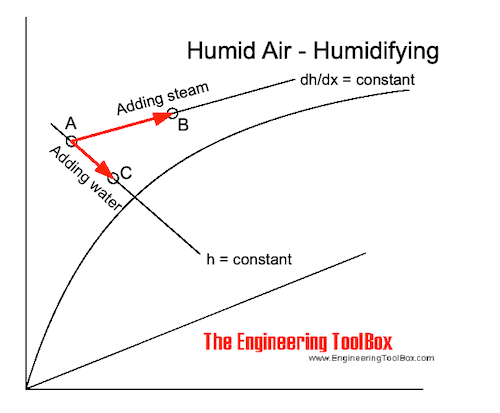
Air - Humidifying by Adding Steam or Water
Air can be humidified by adding water or steam.
Air can be humidified by
- adding water, or
- adding water vapor - or steam
Humidifying Air by adding Water
If water is added to the air without any heat supply the state of the air changes adiabatic along a constant enthalpy line - h - in the Mollier or psychrometric chart . The dry temperature of the air decreases as indicated in the process from A to C in the Mollier diagram above.
The amount of water required to change the specific humidity can be calculated as
mw = v ρ (xC - xA) (1)
where
mw = mass of added water (kg/s)
v = volume air flow (m3/s)
ρ = density of air (kg/m3) - varies with temperature, 1.204 kg/m3 at 20 oC and 1 atm
x = humidity ratio (kgh2o/kgdry_air )
If water is added at the same temperature as the air the enthalpy change is zero.
Example - Humidifying Air by adding Water
An airflow of 3000 m3/h at 25 oC and 10% relative humidity (A) is humidified to 60% relative humidity (C) by adding water through spray nozzles.
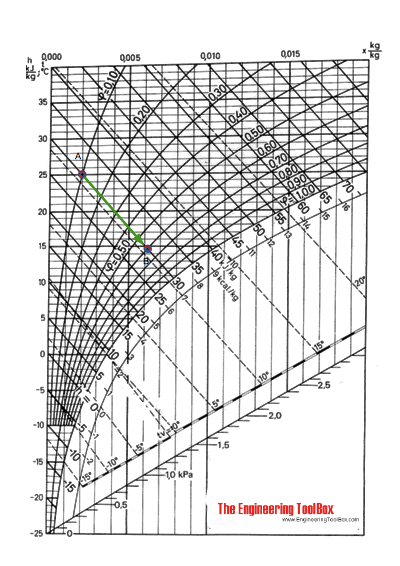
Using the Mollier diagram following the constant enthalpy line 30 kJ/kg from A to 60% relative humidity, the state at C can be found at 14.7 oC .
The humidity ratio at (A) is 0.002 kg/kg and at (C) 0.0062 kg/kg .
The amount of water:
mw = ((3000 m3/h) / (3600 s/h)) (1.184 kg/m3) ( (0.0062 kg/kg) - (0.002 kg/kg))
= 0.0041 kg/s
= 14.9 kg/h
Humidifying Air by adding Steam
If steam is added to the air the state will change along a constant dh/dx - line for steam as indicated in process A to B in the Mollier diagram above.
When adding saturated steam at atmospheric pressure the constant line dh/dx = 2502 kJ/kg (the evaporation heat of water at atmospheric pressure). When adding saturated steam at atmospheric pressure the temperature rise is very small - in general less than 1 oC. For practical purposes the process of adding saturated steam at atmospheric pressure approximates the horizontal temperature line.
The water vapor added can be calculated using (1).
The enthalpy added can be estimated by using the Mollier diagram or a psyhrometric chart.
Example - Humidifying Air by adding Steam
An airflow of 3000 m3/h at 25 oC and 10% relative humidity (A) is humidified to 60% relative humidity (B) by adding saturated steam at atmospheric pressure.
Using the Mollier diagram the process from (A) to (B) can be approximated by following the constant temperature line 25 oC to 60% relative humidity and approximately 25.5 oC (a temperature increase less than 1 oC).
Note! - by adding enough steam the state of air (B) is moved to the saturation line where the air is fully saturated. By adding even more steam the process starts following the saturation line - moisture content and dry bulb temperature will increase - and droplets or fog may be introduced in the moist air.
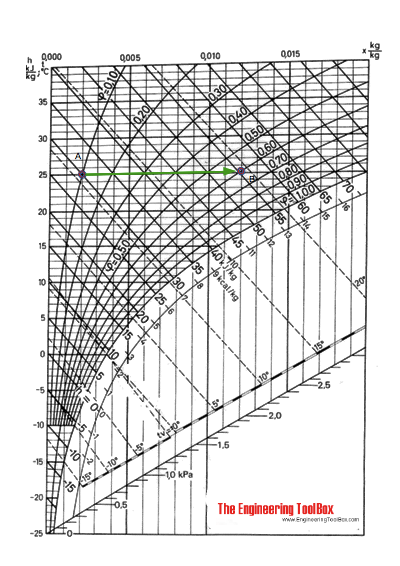
The humidity ratio at (A) is 0.002 kg/kg and at (B) 0.012 kg/kg .
The amount of water added can be calculated as:
mw = ((3000 m3/h) / (3600 s/h)) (1.184 kg/m3) ( (0.012 kg/kg) - (0.002 kg/kg))
= 0.01 kg/s
= 35.5 kg/h
The enthalpy change can be estimated from the Mollier diagram. The enthaply at (A) is 30 kJ/kg and at (B) 55 kJ/kg. The enthalpy difference is
dh = (55 kJ/kg) - (30 kJ/kg)
= 25 kJ/kg
The total heat added by the steam can be calculated as:
q = ((3000 m3/h) / (3600 s/h)) (1.184 kg/m3) (55 kJ/kg - 30 kJ/kg)
= 24.7 (kJ/s, kW)
Example - Humidifying Air with Steam in the Psychrometric Chart
The chart below indicates a similar process of humidifying air with steam in the psychrometric chart.
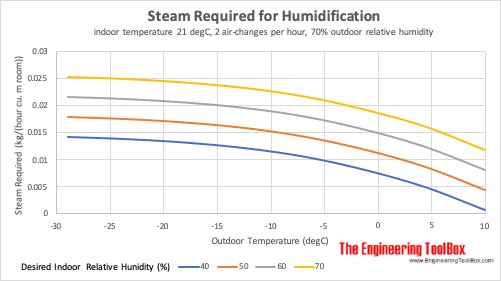
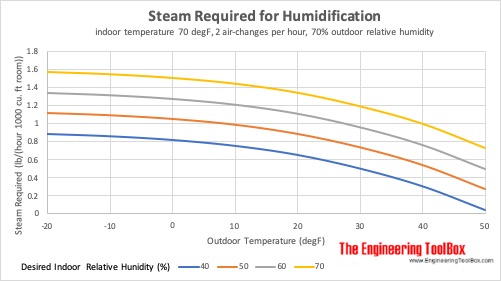
Steam Required for Humidification at different Outdoor Temperatures
Steam required for humidification of indoor space at 21°C (70°F), 2 air-changes per hour and 70% outdoor relative humidity: