Densities of Aqueous Solutions of Inorganic Sodium Salts
Changes in density of aqueous solutions with changes in concentration at 20°C. Density of inorganic sodium salts in water is plotted as function of wt%, mol/kg water and mol/l solution.
Be aware of the concentration units in the figures:
wt%: Mass of solute/total mass of solution ×100%
mol/kg: Molality = moles of solute/kg of water
mol/liter: Molarity = moles of solute/liter of solution
Values are tabulated below the figures.
See also density of aqueous solutions of inorganic chlorides, inorganic potassium salts, some other inorganic substances, organic acids and organic substances as sugars and alcohols.
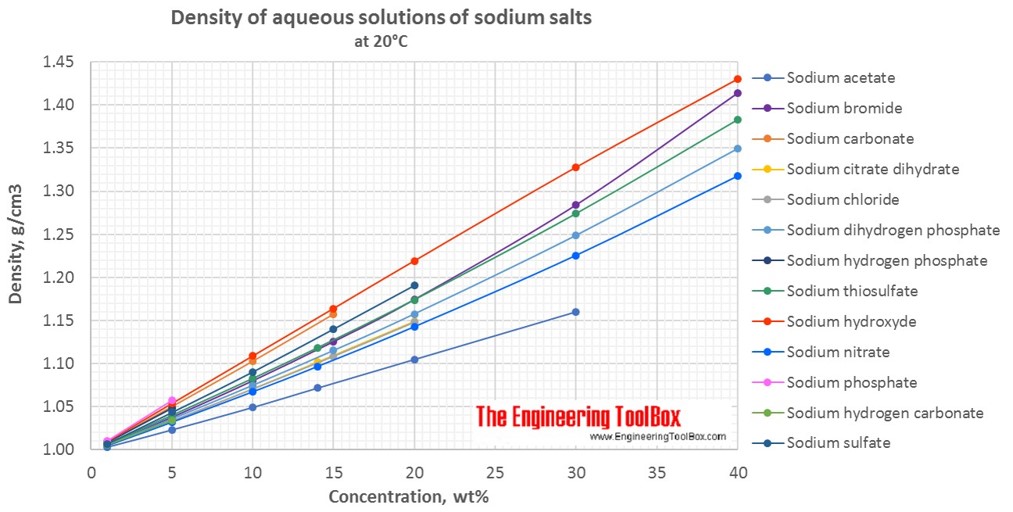
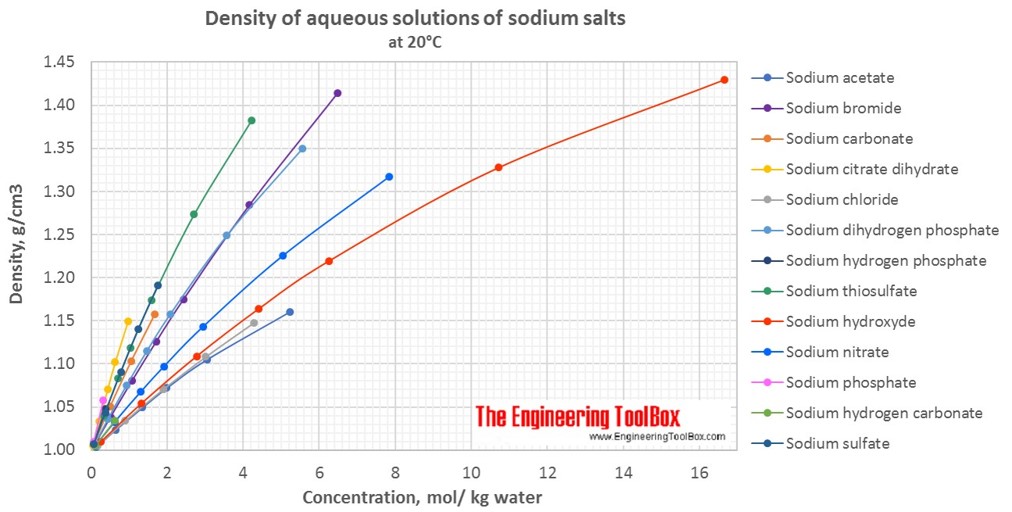

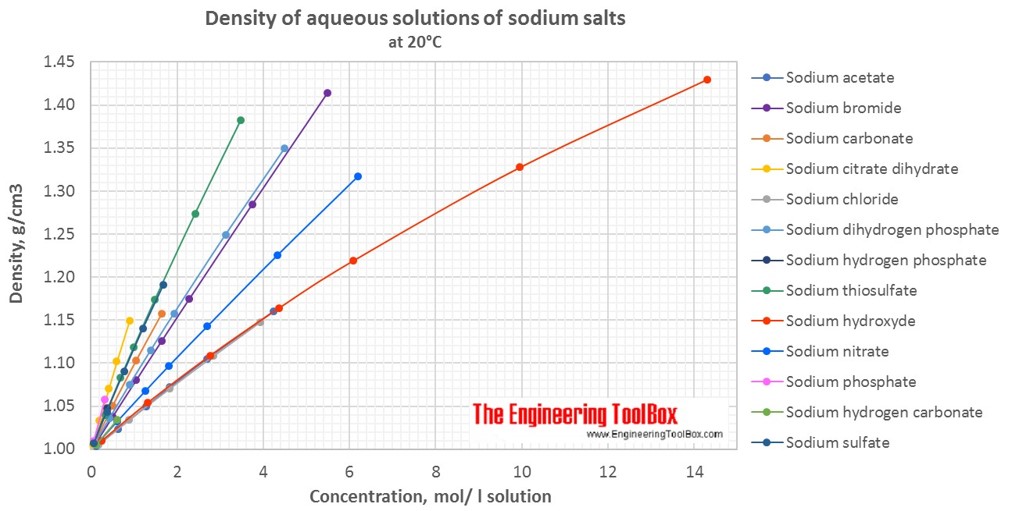
Density of aqueous solutions at 20°C, given as g/cm3:
For full table - rotate the screen!
| Mass% | Sodium acetate | Sodium bromide | Sodium carbonate | Sodium chloride | Sodium citrate dihydrate | Sodium dihydrogen phosphate | Sodium hydrogen carbonate | Sodium hydrogen phosphate | Sodium hydroxyde | Sodium nitrate | Sodium phosphate | Sodium sulfate | Sodium thiosulfate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.0034 | 1.006 | 1.0086 | 1.0053 | 1.0049 | 1.0056 | 1.0054 | 1.0082 | 1.0095 | 1.005 | 1.01 | 1.0071 | 1.0065 |
| 5 | 1.0234 | 1.038 | 1.0502 | 1.034 | 1.0331 | 1.0358 | 1.0337 | 1.048 | 1.0538 | 1.0322 | 1.0579 | 1.0436 | 1.0399 |
| 10 | 1.0495 | 1.0803 | 1.1029 | 1.0707 | 1.0708 | 1.0747 | 1.1089 | 1.0674 | 1.0905 | 1.0827 | |||
| 20 | 1.1050 | 1.1745 | 1.1478 | 1.1492 | 1.1576 | 1.2192 | 1.1429 | 1.1907 | 1.174 | ||||
| 30 | 1.1602 | 1.2842 | 1.2488 | 1.3277 | 1.2256 | 1.2739 | |||||||
| 40 | 1.4138 | 1.3493 | 1.4299 | 1.3175 | 1.3827 | ||||||||
| Density at 20°C, given as g/cm3 | |||||||||||||
Conversion of the concentration from mass% to mol/kg (moles of solute/kg of water = molality):
For full table - rotate the screen!
| Mass% | Sodium acetate | Sodium bromide | Sodium carbonate | Sodium chloride | Sodium citrate dihydrate | Sodium dihydrogen phosphate | Sodium hydrogen carbonate | Sodium hydrogen phosphate | Sodium hydroxyde | Sodium nitrate | Sodium phosphate | Sodium sulfate | Sodium thiosulfate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.123 | 0.098 | 0.095 | 0.173 | 0.039 | 0.084 | 0.12 | 0.071 | 0.253 | 0.119 | 0.062 | 0.071 | 0.064 |
| 5 | 0.642 | 0.512 | 0.497 | 0.901 | 0.204 | 0.439 | 0.627 | 0.371 | 1.316 | 0.619 | 0.321 | 0.371 | 0.333 |
| 10 | 1.354 | 1.08 | 1.048 | 1.901 | 0.431 | 0.926 | 2.778 | 1.307 | 0.782 | 0.703 | |||
| 20 | 3.047 | 2.43 | 4.278 | 0.969 | 2.084 | 6.25 | 2.941 | 1.76 | 1.581 | ||||
| 30 | 5.224 | 4.165 | 3.572 | 10.715 | 5.042 | 2.711 | |||||||
| 40 | 6.479 | 5.557 | 16.668 | 7.844 | 4.216 | ||||||||
| Molality at 20°C, given as mol/kg water | |||||||||||||
Conversion of the concentration from mass% to mol/liter (moles of solute/liter of solution = molarity):
For full table - rotate the screen!
| Mass% | Sodium acetate | Sodium bromide | Sodium carbonate | Sodium chloride | Sodium citrate dihydrate | Sodium dihydrogen phosphate | Sodium hydrogen carbonate | Sodium hydrogen phosphate | Sodium hydroxyde | Sodium nitrate | Sodium phosphate | Sodium sulfate | Sodium thiosulfate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.122 | 0.098 | 0.095 | 0.172 | 0.039 | 0.084 | 0.12 | 0.071 | 0.252 | 0.118 | 0.062 | 0.071 | 0.064 |
| 5 | 0.624 | 0.504 | 0.495 | 0.885 | 0.2 | 0.432 | 0.615 | 0.369 | 1.317 | 0.607 | 0.323 | 0.367 | 0.329 |
| 10 | 1.279 | 1.05 | 1.041 | 1.832 | 0.415 | 0.896 | 2.772 | 1.256 | 0.768 | 0.685 | |||
| 20 | 2.694 | 2.283 | 3.928 | 0.891 | 1.93 | 6.096 | 2.6989 | 1.677 | 1.485 | ||||
| 30 | 4.243 | 3.744 | 3.123 | 9.958 | 4.326 | 2.417 | |||||||
| 40 | 5.496 | 4.499 | 14.3 | 6.2 | 3.48 | ||||||||
| Molarity at 20°C, given as mol/l solution | |||||||||||||



