Ethylene Glycol Heat-Transfer Fluid Properties
Properties like freezing point, viscosity, specific gravity and specific heat of ethylene glycol based heat-transfer fluids, or brines.
Ethylene Glycol based water solutions are common in heat-transfer applications where the temperature in the heat transfer fluid can be below 32 oF (0 oC) . Ethylene glycol is also commonly used in heating applications that temporarily may not be operated (cold) in surroundings with freezing conditions - such as cars and machines with water cooled engines.
Ethylene Glycol is the most common antifreeze fluid for standard heating and cooling applications. Ethylene glycol should be avoided if there is a slightest chance of leakage to potable water or food processing systems. Instead solutions based on propylene glycol are commonly used.
Specific heat, viscosity and specific weight of a water and ethylene glycol solution vary significantly with the percent of ethylene glycol and the temperature of the fluid. Properties differs so much from clean water that heat transfer systems with ethylene glycol should be calculated thoroughly for actual temperature and solution.
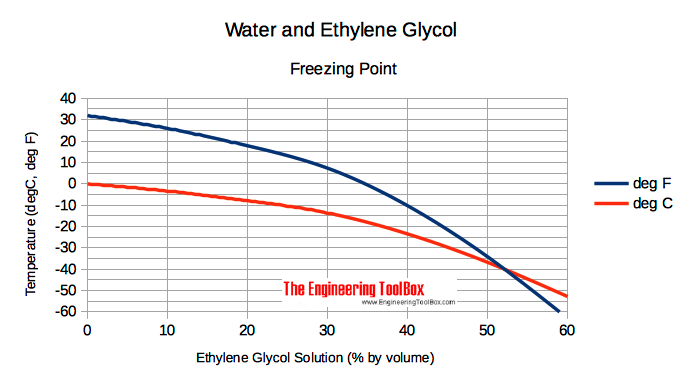
Freezing Point of Ethylene Glycol based Water Solutions
Freezing points of ethylene glycol based water solutions at various temperatures are indicated below
| Ethylene Glycol Solution ( % by volume ) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 80 | 90 | 100 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Freezing Point | ( oF) | 32 | 25.9 | 17.8 | 7.3 | -10.3 | -34.2 | -63 | ≈ -51 | ≈ -22 | 9 |
| (oC) | 0 | -3.4 | -7.9 | -13.7 | -23.5 | -36.8 | -52.8 | ≈ -46 | ≈ -30 | -12.8 | |
Due to possible slush creation, ethylene glycol and water solutions should not be used in conditions close to freezing points.
Dynamic Viscosity of Ethylene Glycol based Water Solutions
Dynamic viscosity - μ - of ethylene glycol based water solutions at various temperatures are indicated below
| Temperature | Dynamic Viscosity - μ - (centiPoise) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ethylene Glycol Solution (% by volume) | ||||||||
| ( oF) | (oC) | 25 | 30 | 40 | 50 | 60 | 65 | 100 |
| 0 | -17.8 | 1) | 1) | 15 | 22 | 35 | 45 | 310 |
| 40 | 4.4 | 3 | 3.5 | 4.8 | 6.5 | 9 | 10.2 | 48 |
| 80 | 26.7 | 1.5 | 1.7 | 2.2 | 2.8 | 3.8 | 4.5 | 15.5 |
| 120 | 48.9 | 0.9 | 1 | 1.3 | 1.5 | 2 | 2.4 | 7 |
| 160 | 71.1 | 0.65 | 0.7 | 0.8 | 0.95 | 1.3 | 1.5 | 3.8 |
| 200 | 93.3 | 0.48 | 0.5 | 0.6 | 0.7 | 0.88 | 0.98 | 2.4 |
| 240 | 115.6 | 2) | 2) | 2) | 2) | 2) | 2) | 1.8 |
| 280 | 137.8 | 2) | 2) | 2) | 2) | 2) | 2) | 1.2 |
- below freezing point
- above boiling point
Note! The dynamic viscosity of an ethylene glycol based water solution is increased compared with the dynamic viscosity of clean water . As a consequence the head loss (pressure loss) in the a piping system with ethylene glycol is increased compared to clean water.
Specific Gravity of Ethylene Glycol based Water Solutions
Specific gravity - SG - of ethylene glycol based water solutions at various temperatures are indicated below
| Temperature | Specific Gravity - SG - | |||||||
|---|---|---|---|---|---|---|---|---|
| Ethylene Glycol Solution (% by volume) | ||||||||
| ( oF) | (oC) | 25 | 30 | 40 | 50 | 60 | 65 | 100 |
| -40 | -40 | 1) | 1) | 1) | 1) | 1.12 | 1.13 | 1) |
| 0 | -17.8 | 1) | 1) | 1.08 | 1.10 | 1.11 | 1.12 | 1.16 |
| 40 | 4.4 | 1.048 | 1.057 | 1.07 | 1.088 | 1.1 | 1.11 | 1.145 |
| 80 | 26.7 | 1.04 | 1.048 | 1.06 | 1.077 | 1.09 | 1.095 | 1.13 |
| 120 | 48.9 | 1.03 | 1.038 | 1.05 | 1.064 | 1.077 | 1.082 | 1.115 |
| 160 | 71.1 | 1.018 | 1.025 | 1.038 | 1.05 | 1.062 | 1.068 | 1.1 |
| 200 | 93.3 | 1.005 | 1.013 | 1.026 | 1.038 | 1.049 | 1.054 | 1.084 |
| 240 | 115.6 | 2) | 2) | 2) | 2) | 2) | 2) | 1.067 |
| 280 | 137.8 | 2) | 2) | 2) | 2) | 2) | 2) | 1.05 |
- below freezing point
- above boiling point
Note! The specific gravity of ethylene glycol based water solutions are increased compared with specific gravity of clean water .
Densities of Ethylene Glycol based Water Solutions
Turn the screen to see the whole table.
| Mass Fraction of Ethylene Glycol in Solution | Density - ρ - (kg/m3 ) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature - t - (oC) | ||||||||||||
| -48 | -35 | -25 | -14 | -8 | -4 | 0 | 20 | 40 | 60 | 80 | 100 | |
| 0 | 1000 | 998 | 992 | 983 | 972 | 958 | ||||||
| 0.1 | 1019 | 1018 | 1014 | 1008 | 1000 | 992 | 984 | |||||
| 0.2 | 1038 | 1037 | 1036 | 1030 | 1022 | 1014 | 1005 | 995 | ||||
| 0.3 | 1058 | 1056 | 1055 | 1054 | 1046 | 1037 | 1027 | 1017 | 1007 | |||
| 0.4 | 1080 | 1077 | 1075 | 1073 | 1072 | 1063 | 1052 | 1041 | 1030 | 1018 | ||
| 0.5 | 1103 | 1100 | 1096 | 1093 | 1092 | 1090 | 1079 | 1067 | 1055 | 1042 | 1030 | |
| 0.6 | 1127 | 1124 | 1120 | 1115 | 1112 | 1110 | 1107 | 1095 | 1082 | 1068 | 1055 | 1042 |
Example - Expansion Volume in a Heating System with Ethylene Glycol
A heating system with liquid volume 0.8 m3 is freeze protected with 50% (by mass, mass fraction 0.5) ethylene glycol. The installation temperature of the system is down to 0 oC and the maximum medium operation temperature is 80 oC .
From the table above we see that the density of the solution at installation temperature can be as high as 1090 kg/m3 - and the medium density at operation temperature can be as low as 1042 kg/m3 .
The mass of the liquid at installation can be calculated as
m inst = ρ inst V inst (1)
= (1090 kg/m3 ) (0.8 m3 )
= 872 kg
where
m inst = mass of liquid at installation (kg)
ρ inst = density at installation (kg/m3 )
V inst = liquid volume at installation (m3 )
Mass of the liquid in the system during operation will be same as the mass in system during installation
m inst = m op (2)
= ρ op V op
where
m op = mass of liquid at operation (kg)
ρ op = density at operation (kg/m3 )
V op = liquid volume at operation (m3 )
(2) can be modified to calculate liquid operation volume as
V op = m inst / ρ op (2b)
= (872 kg) / ( 1042 kg/m3 )
= 0.837 m3
The required expansion volume to avoid pressure can be calculated as
ΔV = V op - V inst (3)
= (0.837 m3 ) - (0.8 m3 )
= 0.037 m3
= 37 liter
where
ΔV = expansion volume (m3 )
Expansion volume can be calculated as
ΔV = ( ρ inst / ρ op - 1 ) V inst (4)
Specific Heat of Ethylene Glycol based Water Solutions
Specific Heat - cp - of ethylene glycol based water solutions at various temperatures are indicated below
Turn the screen to the whole table.
| Ethylene Glycol Solution (% by weight) | Specific Heat - cp (Btu/lb oF) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | ||||||||||||||||
| -50 | -40 | -30 | -20 | -10 | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |
| 0 | 1.0038 | 1.0018 | 1.0004 | 0.99943 | 0.99902 | 0.99913 | 0.99978 | 1.0009 | 1.0026 | 1.0049 | 1.0076 | |||||
| 10 | 0.97236 | 0.97422 | 0.97619 | 0.97827 | 0.98047 | 0.98279 | 0.98521 | 0.98776 | 0.99041 | 0.99318 | 0.99607 | |||||
| 20 | 0.93576 | 0.93976 | 0.94375 | 0.94775 | 0.95175 | 0.95574 | 0.95974 | 0.96373 | 0.96773 | 0.97173 | 0.97572 | |||||
| 30 | 0.89373 | 0.89889 | 0.90405 | 0.90920 | 0.91436 | 0.91951 | 0.92467 | 0.92982 | 0.93498 | 0.94013 | 0.94529 | 0.95044 | ||||
| 40 | 0.84605 | 0.85232 | 0.85858 | 0.86484 | 0.87111 | 0.87737 | 0.88364 | 0.88990 | 0.89616 | 0.90243 | 0.90869 | 0.91496 | 0.92122 | |||
| 50 | 0.79288 | 0.80021 | 0.80753 | 0.81485 | 0.82217 | 0.82949 | 0.83682 | 0.84414 | 0.85146 | 0.85878 | 0.86610 | 0.87343 | 0.88075 | 0.88807 | ||
| 60 | 0.72603 | 0.73436 | 0.74269 | 0.75102 | 0.75935 | 0.76768 | 0.77601 | 0.78434 | 0.79267 | 0.80100 | 0.80933 | 0.81766 | 0.82599 | 0.83431 | 0.84264 | 0.85097 |
| 70 | 0.67064 | 0.67992 | 0.68921 | 0.69850 | 0.70778 | 0.71707 | 0.72636 | 0.73564 | 0.74493 | 0.75422 | 0.76350 | 0.77279 | 0.78207 | 0.79136 | 0.80065 | 0.80993 |
| 80 | 0.61208 | 0.62227 | 0.63246 | 0.64265 | 0.65285 | 0.66304 | 0.67323 | 0.68343 | 0.69362 | 0.70381 | 0.71401 | 0.72420 | 0.73439 | 0.74458 | 0.75478 | 0.76497 |
| 90 | 0.58347 | 0.59452 | 0.60557 | 0.61662 | 0.62767 | 0.63872 | 0.64977 | 0.66082 | 0.67186 | 0.68291 | 0.69396 | 0.70501 | 0.71606 | |||
| 100 | 0.53282 | 0.54467 | 0.55652 | 0.56838 | 0.58023 | 0.59209 | 0.60394 | 0.61579 | 0.62765 | 0.63950 | 0.65136 | 0.66321 | ||||
- Freezing point 100% ethylene glycol at atmospheric pressure is -12.8 oC (9 oF)
- 1 Btu/(lbm oF) = 4,186.8 J/(kg K) = 1 kcal/(kg oC)
Note! The specific heat of ethylene glycol based water solutions are less than the specific heat of clean water. For a heat transfer system with ethylene glycol the circulated volume must be increased compared to a system only with water.
In a 50% solution with operational temperatures above 36 oF the specific heat capacity is decreased with approximately 20% . The reduced heat capacity must be compensated by circulating more fluid.
Note! The density of ethylene glycol is higher than water - check the specific gravity (SG) table above, so the net impact on the heat transport capacity is reduced. Example - the specific heat of an ethylene glycol water solution 50% / 50% is 0.815 at 80 oF (26.7 oC). Specific gravity at the same conditions is 1.077. The net impact can be estimated to 0.815 * 1.077 = 0.877.
Automobile antifreeze solutions should not be used in HVAC systems because they contain silicates that may cause fouling. Silicates in automobile antifreeze are used to protect aluminum engine parts.
Note! Distilled or deionized water should be used for ethylene glycol solutions. City water may be treated with chlorine which is corrosive.
Systems for automatic makeup water should not be used since a leakage would contaminate the environment and dilute the antifreeze protection of the system.
Boiling Points Ethylene Glycol Solutions
For full table with Boiling Points - rotate the screen!
| Ethylene Glycol Solution (% by volume) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling Point | ( oF) | 212 | 214 | 216 | 220 | 220 | 225 | 232 | 245 | 260 | 288 | 386 |
| (oC) | 100 | 101.1 | 102.2 | 104.4 | 104.4 | 107.2 | 111.1 | 118 | 127 | 142 | 197 | |
Increase in Flow required for a 50% Ethylene Glycol Solution
Increase in circulated flow for 50% ethylene glycol solutions compared with clean water are indicated in the table below
| Fluid Temperature | Flow Increase (%) | |
|---|---|---|
| ( oF) | (oC) | |
| 40 | 4.4 | 22 |
| 100 | 37.8 | 16 |
| 140 | 60.0 | 15 |
| 180 | 82.2 | 14 |
| 220 | 104.4 | 14 |
Pressure Drop Correction and Combined Pressure Drop and Volume Flow Correction for 50% Ethylene Glycol Solution
Pressure drop correction and combined pressure drop and flow increase correction for 50% ethylene glycol solutions compared with clean water are indicated in the table below
| Fluid Temperature | Pressure Drop Correction with Flow Rates Equal (%) | Combined Pressure Drop and Flow Rate Correction (%) | |
|---|---|---|---|
| ( oF) | (oC) | ||
| 40 | 4.4 | 45 | 114 |
| 100 | 37.8 | 10 | 49 |
| 140 | 60.0 | 0 | 32 |
| 180 | 82.2 | -6 | 23 |
| 220 | 104.4 | -10 | 18 |
Related Topics
-
Air Conditioning Systems
Design of Air Conditioning systems - heating, cooling and dehumidification of indoor air for thermal comfort. -
Boiling Points of Fluids
Boiling points of elements, products and chemical species at varying conditions. -
Densities
Densities of solids, liquids and gases. Definitions and convertion calculators. -
Material Properties
Properties of gases, fluids and solids. Densities, specific heats, viscosities and more. -
Viscosities
Viscosities of products and chemical species at varying conditions.
Related Documents
-
Antifreeze - Ethylene Glycol Volume vs. Temperature Rating
Freeze protecting of cooling systems - temperature rating vs. required amount of antifreeze. -
Antifreeze - Ethylene Glycol vs. Propylene Glycol
Comparing ethylene glycol and propylene glycol antifreeze properties. -
Boiling Liquids - Max Pumping Flow Velocities
Recommended max flow velocity on the delivery (pressure) side when pumping boiling liquids. -
Calcium Chloride Water Solutions
Freezing point, density, specific heat and dynamic viscosity of Calcium Chloride Water coolants. -
Dowtherm A
Physical properties of Dowtherm A. -
Ethanol Freeze Protected Water Solutions
Freezing temperature and flash points for ethanol based water solutions or brines. -
Ethanol Water Mixtures - Densities vs. Temperature
Density of Ethyl Alcohol aqueous solutions. -
Ethylene - Density and Specific Weight vs. Temperature and Pressure
Online calculator, figures and tables showing density and specific weight of ethylene, C2H4, at varying temperature and pressure - Imperial and SI Units. -
Ethylene Gas - Specific Heat vs. Temperature
Specific heat of Ethylene Gas - C2H4 - temperatures ranging 175 - 900 K. -
Freeze Protection of Water based Heat Transfer Fluids
Comparing antifreezes used in water based heat transfer fluids or brines. -
Freezing Mixtures and Cooling Agents
Freezing mixtures, cooling agents and freezing points. -
Glycerine - Boiling and Freezing Points
Boiling and freezing points of glycerine aqueous solutions. -
Hot Water Expansion Tanks - Sizing
Required hot water expansion volume in open, closed and diaphragm tanks. -
Hot Water Heating Systems - Online Design Application
Free online design tool for designing hot water heating systems - metric units. -
Hot Water Heating Systems - Online Design Application, Imperial Units
Online design tool for hot water heating systems. -
Hot Water Heating Systems - Pressure Loss in Steel Pipes
Pressure loss nomogram for hot water steel pipes. -
Hydrocarbons, Alcohols and Acids - Boiling points
Boiling temperatures (°C and °F) with varying carbon numbers up to C33. -
Isopropanol (2-Propanol) based Freeze Protected Water Solutions
Freezing and flash points of isopropanol (2-Propanol) based water solutions or brines. -
Melting points of Hydrocarbons, Alcohols and Acids
Melting temperature (°C and °F) with carbon number up to C33. -
Methanol Freeze Protected Heat Transfer Liquids
Freezing and flash points for methanol or methyl based heat-transfer fluids or brines. -
Piston Engines - Compression Ratios
Cylinder volume and compression ratios in piston engines. -
Propylene Glycol based Heat-Transfer Fluids
Freezing points of propylene glycol based heat-transfer fluids suitable for the food processing industry. -
Secondary Coolants - Properties
Comparing properties like specific gravity, freezing points and viscosity for secondary coolants like calcium chloride, sodium chloride, ethylene glycol and propylene glycol. -
Snow Melting Systems
Sizing snow melting systems - water and antifreeze. -
Sodium Chloride Water Solutions
Freezing point, density, specific heat and dynamic viscosity of Sodium Chloride and Water coolant. -
Storm-Water Runoff Coefficients vs. Surface
Storm-water runoff coefficients for various surfaces -
Volumetric (Cubic) Thermal Expansion
Volumetric temperature expansion calculator.




