Glycerine - Boiling and Freezing Points
Boiling and freezing points of glycerine aqueous solutions.
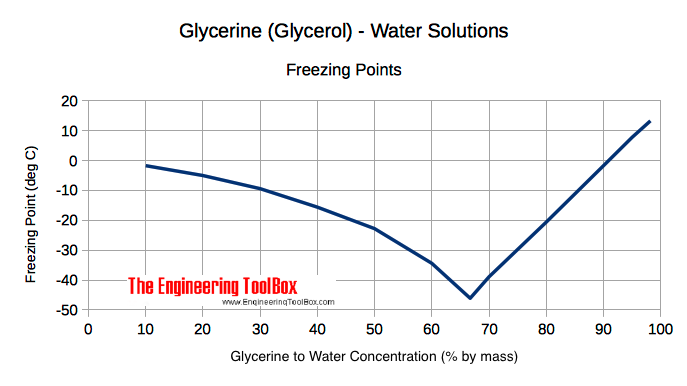
The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. The freezing points are reduced until glycerine concentration is 66.7 % (mass). Increasing the glycerine concentration above 66.7 % will increase the freezing point as indicated below.
| Glycerine to Water Concentration (% by mass, weight) | Specific Gravity (at 60oF, 15.6oC) | Freezing Point | Boiling Point | ||
|---|---|---|---|---|---|
| (oF) | (oC) | (oF) | (oC) | ||
| 98.2 | 1.261 | 56 | 13.3 | 554 | 290 |
| 95 | 1.253 | 46 | 7.8 | 332 | 167 |
| 90 | 1.240 | 29 | -1.7 | 281 | 138 |
| 80 | 1.213 | -5 | -20.6 | 250 | 121 |
| 70 | 1.185 | -38 | -38.9 | 237 | 114 |
| 66.7 | 1.178 | -51 | -46.1 | 234 | 112 |
| 60 | 1.157 | -30 | -34.4 | 228 | 109 |
| 50 | 1.129 | -9 | -22.8 | 223 | 106 |
| 40 | 1.102 | 4 | -15.6 | 219 | 104 |
| 30 | 1.075 | 15 | -9.4 | 217 | 103 |
| 20 | 23 | -5.0 | |||
| 10 | 28.9 | -1.7 | |||
Related Topics
-
Boiling Points of Fluids
Boiling points of elements, products and chemical species at varying conditions. -
Material Properties
Properties of gases, fluids and solids. Densities, specific heats, viscosities and more. -
Melting and Freezing Points
Melting and freezing points of elements and chemical species at varying conditions.
Related Documents
-
Alcohols and Carboxylic Acids - Physical Data
Molweight, melting and boiling point, density, pKa-values, as well as number of carbon and hydrogen atoms in molecules are given for 150 different alcohols and acids. -
Ethylene Glycol Heat-Transfer Fluid Properties
Properties like freezing point, viscosity, specific gravity and specific heat of ethylene glycol based heat-transfer fluids, or brines. -
Heat Transfer Coefficients in Heat Exchanger Surface Combinations
Average overall heat transmission coefficients for fluid and surface combinations like Water to Air, Water to Water, Air to Air, Steam to Water and more. -
Hydrocarbons - Physical Data
Molweight, melting and boiling point, density, flash point and autoignition temperature, as well as number of carbon and hydrogen atoms in each molecule for 200 different hydrocarbons. -
Hydrocarbons, Alcohols and Acids - Boiling points
Boiling temperatures (°C and °F) with varying carbon numbers up to C33. -
Isopropanol (2-Propanol) based Freeze Protected Water Solutions
Freezing and flash points of isopropanol (2-Propanol) based water solutions or brines. -
Liquids and Gases - Boiling Points
Boiling temperatures for common liquids and gases - acetone, butane, propane and more. -
Melting points of Hydrocarbons, Alcohols and Acids
Melting temperature (°C and °F) with carbon number up to C33. -
Methanol Freeze Protected Heat Transfer Liquids
Freezing and flash points for methanol or methyl based heat-transfer fluids or brines. -
Propylene Glycol based Heat-Transfer Fluids
Freezing points of propylene glycol based heat-transfer fluids suitable for the food processing industry.




