Water-Steam Mollier Diagram
Enthalpy-entropy diagram for water and steam.
The diagram below can be used to determine enthalpy versus entropy of water and steam.
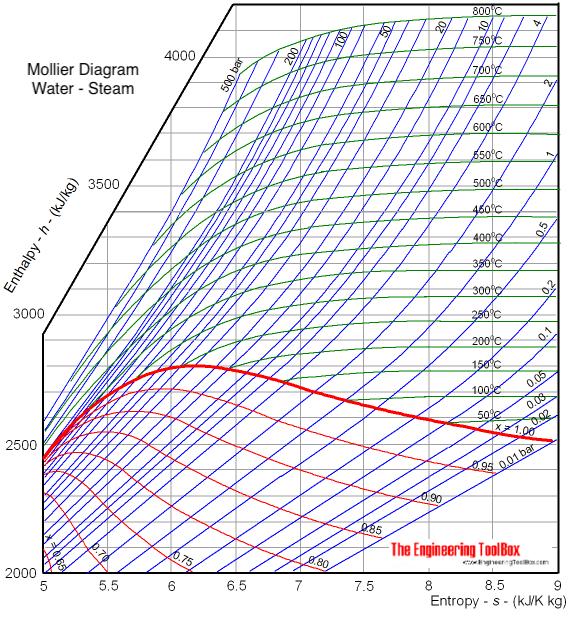
Download and print Mollier Diagram for Water - Steam
The Mollier diagram is useful when analyzing the performance of adiabatic steady-flow processes, such as flow in nozzles, diffusers, turbines and compressors.
See also Water - Enthalpy (H) and Entropy (S) for figures and tabulated values at varying temperatures
Dryness Fraction
The quantity of saturated vapor in unit mass of wet vapor , denoted by x , is referred to as the
dryness fraction, or quality,
of the vapor.
Convert to other Units
- 1 bar = 105 Pa (N/m2) = 0.1 N/mm2 = 10,197 kp/m2 = 10.20 m H2O = 0.9869 atm = 14.50 psi (lbf/in2) = 106 dyn/cm2= 750 mmHg
- 1 kJ/kg = 0.4299 Btu/ lbm = 0.23884 kcal/kg
Example - Enthalpy
The enthalpy in saturated steam (x = 1, the bold red line in the diagram) with pressure 10 bar can be estimated to aprox.
2770 kJ/kg
The value can be verified by using the steam table.



