Naming of Organic Compounds
Nomenclature rules for different groups of organic compounds and functional groups, together with examples of use of the rules.
There are millions of different organic compounds and it is obvious that a systematic way of naming them is necessary. This article gives definitions of the different classes of organic compounds and rules for their naming together with many examples of use of the naming rules.
See also naming of inorganic binary compounds.
Content
(links directly to the compound classes)
General naming principles
Organic compounds follow a certain naming pattern
- Prefix = substituent(s)
- First Name = carbon chain number
- Second Name = type of chain
- Suffix = last name: highest priority functional group
In the naming process we start with the First name:
The core skeleton of an organic compound is called its root or parent chain. This refers to the simple skeleton or backbone of the molecule, upon which all the functional groups and substituents are attached. This is the first component to name and identify .
The root chain is the longest continuing carbon chain in a molecule. Sometimes the root chain will be written in a simple manner and sometimes the chain will wind and twist. But as long as the carbons are connected, this is considered the parent chain.
Then we find the Second name:
The second name comes from the saturation of the parent chain , specifically the presence and location of double and triple bonds. These molecules fall into 3 categories:
- Alkane: Last name -ane
- Alkene: Last name -ene
- Alkyne: Last name -yne
Then we identify prefixes:
Substituents are side chains to the root carbon chain. Simple carbon branches are named similar to carbon parent chains.
- Count the carbon atoms and apply the same designation used above
- Use the ending -yl to imply that this is a substituent
When more than one of the same substituent occurs, you have to use a new prefix to designate how many are present as follows:
- no prefix needed, self understood
- di
- tri
- tetra
- penta
If many functional groups are present in a molecule, the groups with the lowest priority will be treated as substituents, and prefixes indicating the kind of functional groups are used. Prefixes of the different functional groups are given in the Functional group priority list.
Finally, we find the suffix:
Functional groups come in many forms, from the alcohol -OH groups to the carboxyl -CO2H. When faced with a single functional group it becomes the last name of the molecule. When faced with more than one functional group you simply choose the group with the highest priority as the last name. The priority order and the suffixes of the different functional groups are given in the Functional group priority list.
More details about naming of the different classes of organic compounds, functional groups and examples of naming are given in the chapters below.
Alkanes
Alkane: An acyclic saturated hydrocarbon, with the general formula Cn H2n+2 . n can vary from 1 to >> 100. Alkanes are also called paraffins .
Cycloalkane: A one-ring (monocyclic) saturated hydrocarbon, with the general formula Cn H2n . Cycloalkanes are also called naphthenes.
Hydrocarbon: An organic compound consisting entirely of hydrogen and carbon.
Alkanes which form a chain are called normal, straight chain or unbranched hydrocarbons.
The simplest alkane compound is methane , with n=1: CH4
- The names are obtained by adding the suffix -an e to the Greek root of the number of carbon atoms, prefixes given in the table below.
Alkanes with n>3 can form structural isomers, which means that the carbon chain with a given total carbon number n can be branched in different ways. All isomers have the same general formula Cn H2n+2 . Straight chain alkanes with n>3 is therefor called n-alkanes (n-butane, n-pentane etc.) to pinpoint that they are normal or unbranched.
Alkyl: An alkyl group is an alkane substituent missing one hydrogen, with general formula Cn H2n+1 .
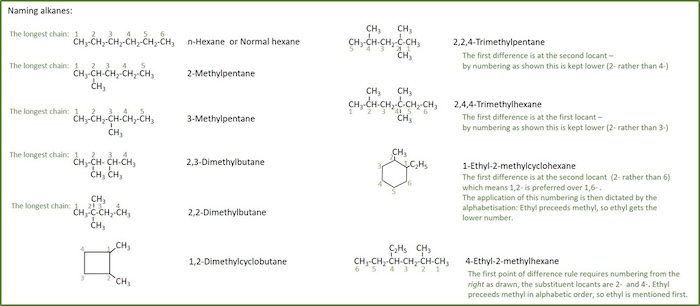
For branched alkanes the following rules are given to name them in a way that precisly describes their structure:
- Find and name the longest continuous carbon chain.
- Identify and name groups attached to this chain. When alkane groups are substituted, they are named by dropping -ane and adding -yl (a substituted CH3-group becomes methyl- and a C2H5-group becomes ethyl).
- Number the chain consecutively, starting at the end nearest a substituent group. Substituents are assigned locants based on this numbering scheme in such a way as to give the lowest locant number at the first time there is a difference.
- Designate the location of each substituent group by an appropriate number and name.
- Assemble the name, listing groups in alphabetical order using the full name (e.g. cyclopropyl before isobutyl). The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing
- If chains of equal length are competing for selection as the parent chain, then the choice goes in series to:
- the chain which has the greatest number of side chains.
- the chain whose substituents have the lowest- numbers.
- the chain having the greatest number of carbon atoms in the smaller side chain.
- the chain having the least branched side chains.
- Cyclic hydrocarbon is designated by the prefix cyclo- which appears directly in front of the base name. The ring is numbered to yield the smallest substituent numbers possible.
Alkenes and alkynes
Alkene: An unsaturated hydrocarbon that contains at least one carbon–carbon double bond, with the general formula Cn H2n (if only one double bond). Alkenes are also called olefin .
Alkyne: An unsaturated hydrocarbon containing at least one carbon—carbon triple bond, with the general formula Cn H2n-2 (if only one triple bond). Alkynes are also called acetylenes .
Cycloalkene: An alkene hydrocarbon which contains a closed ring of carbon atoms, but has no aromatic character, with the general formula Cn H2n-2 . Also called cycloolefin .
The following rules are given to name alkanes and alkyne.
- Double bonds in hydrocarbons are indicated by replacing the name ending -ane with -ene . If there is more than one double bond, the ending is expanded to include a prefix that indicates the number of double bonds present ( - adiene, -atriene etc.).
- Triple bonds are named in a similar way using the ename ending -yne .
- The position of the multiple bonds within the root chain are indicated by placing the numbers of the first carbon of the multiple bonds directly in front of the base name.
- The root chain is numbered so that the multiple bonds have the lowest numbers (double and triple bonds have priority over alkyl and halo substituents).
- When both double and triple bonds are present, numbers as low as possible are given to double and triple bonds even though this may at times give -yne a lower number than -ene . When there is a choice in numbering, the double bonds are given the lowest numbers.
- When both double and triple bonds are present, the -en ending follows the root chain directly and the -yne ending follows the -en ending (notice that the e is left off, -en instead of -ene). The location of the double bonds are indicated before the parent name as before, and the location of the triple bonds are indicated between the -en and -yne suffixes.
- For a branched unsaturated acyclic hydrocarbon, the root chain is the longest carbon chain that contains the maximum number of double and triple bonds. If there are two or more chains competing for selection as the parent chain (chain with the most multiple bonds), the choice goes to
- The chain with the greatest number of carbon atoms,
- If the number of carbon atoms being equal, the chain containing the maximum number of double bonds.
- Alkenes with the R1 -CH=CH-R2unit can exist as cis- and trans- isomers. Identical substituents on the same side of the double bond is named with cis- , while those on opposite sides are called trans- .
- For cyclic alkenes, number through the double bond towards the substituent
- If there is a choice in numbering not previously covered, the parent chain is numbered to give the substituents the lowest number at the first point of difference.

Aromatics
Aromatic hydrocarbon: A cyclic (ring-shaped), planar (flat) molecule with a ring of resonance bonds that exhibits more stability than other geometric or connective arrangements with the same set of atoms. The simplest of the aromatics have 6 carbon atoms and contains 3 double bounds. A one ring aromatic without any substituents is called benzene, with the formula C6 H6 .
Phenyl: A phenyl group is a benzene ring missing one hydrogen, acting as a substituent, with general formula C6 H5.
The nomenclature of aromatics is similar to the nomeclature oof saturated ring systems:
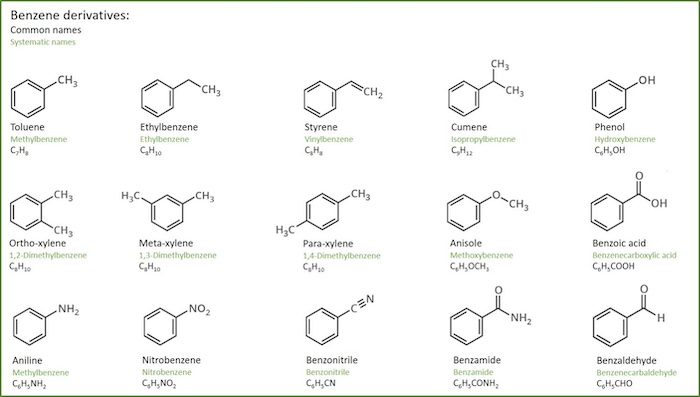
- One way to name these is to use the benzene as the root and add the approriate substituent prefix.
- There are many common simple substituted benzenes with common names that are also used as part of the IUPAC system, examples are given below.
- When there are two (or more) substituents, the relative position of the subsituents must be defined.
-
There are two methods used based on either numerical locants or specific words for the three possible forms:
-
The terms ortho-, meta- or para- (or their singel letter equivalents) are used as prefixes.The words can also be shortened to their first letter, i.e. o-, m- and p-. T hese terms are ONLY used for benzene systems.
- When numerical locants are used, the principal functional group is defined to be at C1. The numerical locant method is also applicable to other aromatic systems.
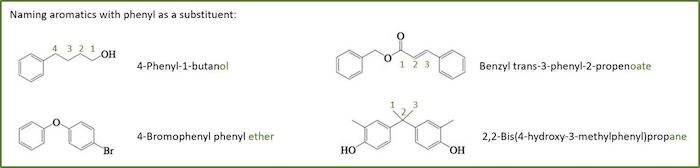
In some cases, the benzene ring needs to be treated as a substituent.
- In these cases, the term phenyl , is used to designate the presence of C6 H5 - as a substituent.
- Take care not to confuse terminology
- phenyl substituent = C6 H5-
- benzyl substituent = C6 H5 CH2-
- phenol a compound = C6 H5 OH
- The method should be used when the benzene ring is a substituent of the root (the root contains the principle functional group).
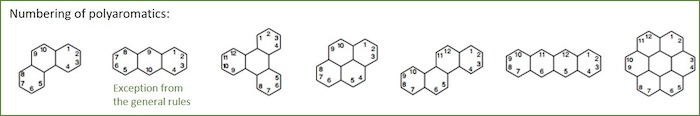
Aromatics with more than two aromatic rings directly connected to each other, are called polyaromatics. Many of these have common names which normally are combined with the IUPAC namenclature. Below are a number of aromatics with more than one aromatic ring given.

For the naming of polyaromatics, IUPAC recommend:
- Names are based on the largest fragment with a trivial name (most to the right in the figure above).
- The simplest attachements are then selected for naming.
- Structures are typically oriented such that
- The greatest number of rings in a row are aligned horizontally
- The maximum number of rings are positioned in the upper right quadrant
- The least number of rings are positioned in the lower left quadrant
- Numbering begins with the uppermost ring the furthest to the right, with the most counterclockwise carbon atom not involved with ring fusion.
- The numbering proceeds clockwise around the structure with hydrogenated carbon atoms.
- The numbering of Anthracene is a "retained exception" to this rule.
Alcohols
Alcohol: An organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom.
Alkanol: An alcohol where the hydroxyl group is bound to an alkyl. If the hydroxyl group is bound to a carbon atom at the end of the alkyl, it is a 1-alkanol, if it is bound to the second carbon, it is a 2-alkanol, etc.
Phenol: An alcohol where the hydroxyl group is bound to a phenyl group, with the formula -C6 H5 OH.
Alcohols are classified according to the number of hydrocarbon substituents bonded to the carbon where the -OH group is attached; primary, secondary and tertiary alcohols.
Naming rules for alcohols:
- Alcohols are named by replacing the suffix -ane with -anol .
- If there is more than one hydroxyl group (-OH), the suffix is expanded to include a prefix that indicates the number of hydroxyl groups present ( - anediol, -anetriol... ).
- The term hydroxy - is used as a prefix.
- The position of the hydroxyl group on the root chain is indicated by placing the number corresponding to the location on the root chain directly in front of the base name (same as alkenes).
- The hydroxyl group takes precedence over alkyl groups and halogen substituents, as well as double bonds, in the numbering of the root chain. For comparison with other groups, see table for functional groups priority.
- When both double bonds and hydroxyl groups are present
- The -en suffix follows the root chain directly and the -ol suffix follows the -en suffix (the e is left off, -en instead of -ene ).
- The location of the double bonds are indicated before the root name as before, and the location of the hydroxyl group is indicated between the -en and -ol suffixes.
- If there is a choice in numbering not previously covered, the root chain is numbered to give the substituents the lowest number at the first point of difference.
Thiols
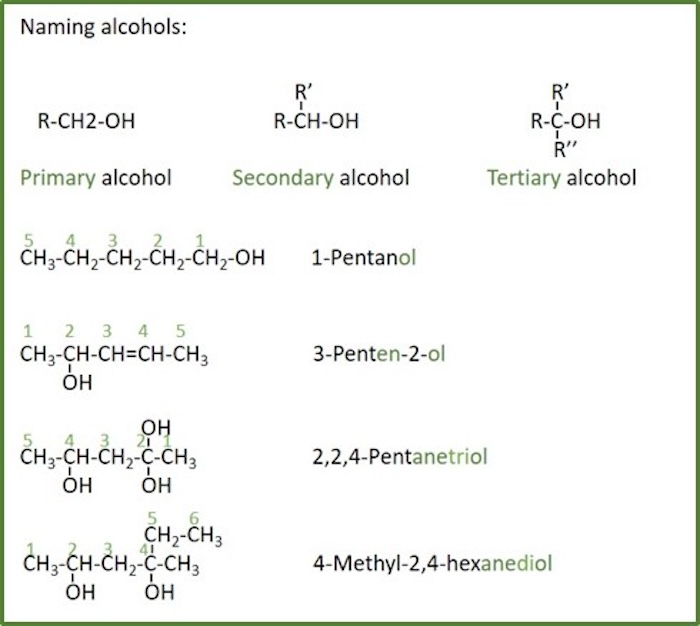
Thiol: An organosulfur compound that contains a carbon-bonded sulfhydryl group (-SH), with the general formula R–SH (where R represents an alkyl or other organic substituent). Thiols are also called mercaptans.
Thiols can be seen as a sulfur analogue to alcohols (sulfur takes the place of oxygen in the hydroxyl group of an alcohol). As the alcohols, thiols are classified according to the number of hydrocarbon substituents bonded to the carbon where the -SH group is attached; primary, secondary and tertiary thiols.
Naming rules for thiols:
- Thiols are named by replacing the suffix -ane with -anthiol .
- If there is more than one sulfhydryl group (-SH), the suffix is expanded to include a prefix that indicates the number of hydroxyl groups present ( - anedithiol, -anetrithiol... ).
- The term sulfanyl- or mercapto- is used as a prefix.
- The position of the sulfhydryl group on the root chain is indicated by placing the number corresponding to the location on the root chain directly in front of the base name (same as alkenes).
- The sulfhydryl group takes precedence after hydroxyl groups (alcohols), but over alkyl groups and halogen substituents, as well as double bonds, in the numbering of the root chain. For comparison with other groups, see table for functional groups priority.
- When both double bonds and sulfhydryl groups are present
- The -en suffix follows the root chain directly and the -thiol suffix follows the -en suffix (the e is left off, -en instead of -ene ).
- The location of the double bonds are indicated before the root name as before, and the location of the hydroxyl group is indicated between the -en and -thiol suffixes.
- If there is a choice in numbering not previously covered, the root chain is numbered to give the substituents the lowest number at the first point of difference.
Amines
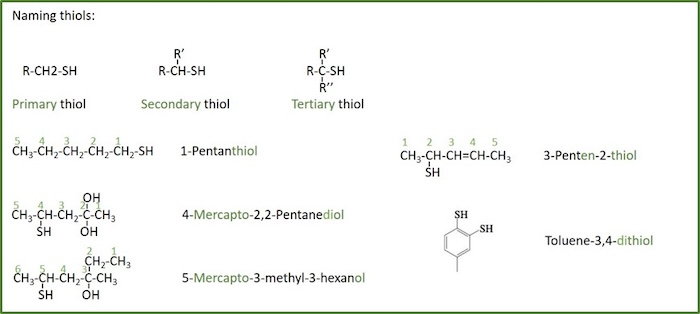
Amine: A compound or functional group that contain a basic nitrogen atom with a lone pair. It can be a primary (R-NH2), a secondary (R,R'-NH) or a tertiary amine (R,R',R''-N), where R, R' and R'' represent an alkyl or other organic substituent.
The nomenclature of amines is complicated by the fact that several different nomenclature systems exist, and there is no clear preference for one over the others. Furthermore, the terms primary, secondary and tertiary are used to classify amines in a completely different manner than they were used for alcohols or alkyl halides. When applied to amines these terms refer to the number of alkyl (or aryl) substituents bonded to the nitrogen atom, whereas in other cases they refer to the nature of an alkyl group. A nitrogen bonded to four alkyl groups will necessarily be positively charged, and is called a quaternary-ammonium cation. For example, (CH3)4 N(+) Br(–) is tetramethylammonium bromide.
In the IUPAC system names amine functions as substituents on the largest alkyl group. The simple -NH substituent found in primary-amines is called an amino- group. For secondary and tertiary amines a compound prefix includes the names of all but the root alkyl group.
The Chemical Abstract Service has adopted a nomenclature system in which the suffix -amine is attached to the root alkyl name.
- For primary amines this is analogous to IUPAC alcohol nomenclature ( -ol suffix).
- The additional nitrogen substituents in secondary and tertiary amines are designated by the prefix N- before the group name.
- The root name is based on the longest chain attached to the N.
- The chain is numbered so as to give the amine unit the lowest possible number.
- The - amine suffix is appended to the appropriate alkyl root or alkana- root.
- The other alkyl group is treated as a substituent, with N as the locant.
- The N locant is listed before numerical locants, e.g. N,2-dimethyl....
A common system for simple amines names each alkyl substituent on nitrogen in alphabetical order, followed by the suffix -amine
Many aromatic and heterocyclic amines are known by unique common names, the origins of which are often unknown by the users. Since these names are not based on a rational system, it is necessary to memorize them.
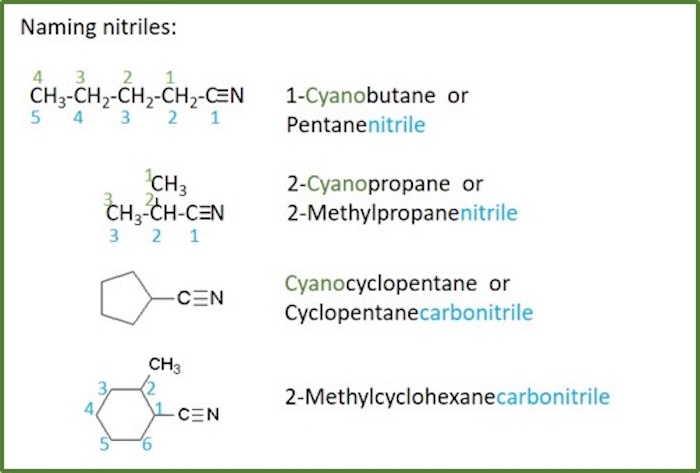
Nitriles
Nitrile: Organic compound that contains an alkyl combined with a - C≡N functional group. Nitriles are also called cyanides , but this is more common in inorganic chemistry.
The nitrile group can also be substitutet to other types of hydrocarbons. If it is connected to e.g. an alkene, it will be a nitroalkene
There are two different ways of naming these compounds:
1. As cyanoalkanes :
- The root name is based on the longest chain containing the cyano group.
- This root give the alkane part of the name.
- The chain is numbered so as to give the cyano group the lowest possible number.
- The cyano- prefix is used in a very similar manner to haloalkane s
- The cyano group takes precedence after acyl halides and amides, but over aldehydes and ketones, in the naming and numbering of the root chain. For comparison with other groups, see table for functional groups priority.
- The cyano nomenclature is most common when the alkyl group is simple.
2. As nitriles :
- The root name is based on the longest chain including the carbon of the nitrile group.
- This root give the alkyl part of the name.
- A - nitrile suffix is placed directly after the alkane part.
- Since the nitrile must be at the end of the chain, it must be C1 and no locant needs to be specified.
- Cycloalkanes are followed by the word -carbonitrile .
Haloalkanes / alkylhalides
Haloalkane: Organic compound that contains an alkyl combined with a halide group. Haloalkenes contain R-X where X can be F, Cl, Br or I. Haloalkanes are also called alkylhalides.
The halide can also be substitutet to other types of hydrocarbons. If it is connected to e.g. an alkene, it will be a haloalkene
There are two different ways of naming these compounds:
1. As haloalkanes (the IUPAC way):
- The root name is based on the longest chain containing the halogen.
- This root give the alkane part of the name.
- The type of halogen defines the halo prefix, e.g. bromo-
- The chain is numbered so as to give the halogen the lowest possible number.
- When there is a doble bond present together with the halide the double bond is given the preference (priority) in the numbering.
- When there are more than one halide atom substituted to the alkyl, the number of halide atoms is indicated with prefixes di-, tri- etc. to the - halo name.
2. As alkyl halides :
- The root name is based on the longest chain containing the halogen.
- This root give the alkyl part of the name.
- The type of halogen defines the halide suffix, e.g . chloride
- The chain is numbered to give the halogen the lowest possible number.
- The alkyl halide nomenclature is most common when the alkyl group is simple.

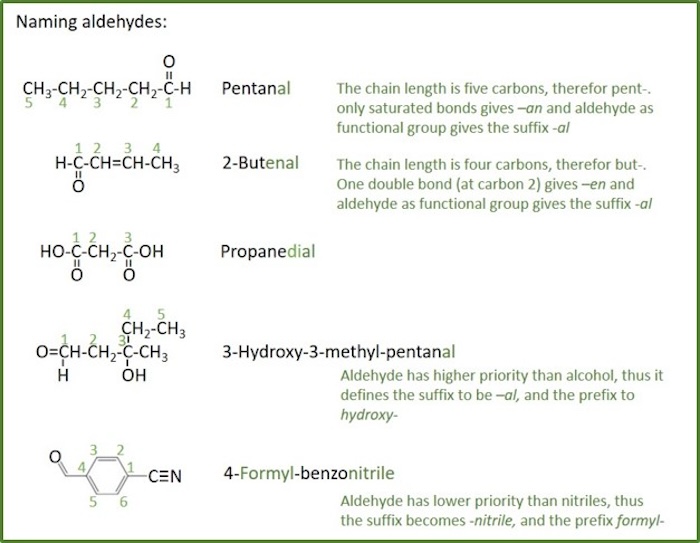
Aldehydes
Aldehyd: An organic compound containing a functional group with the structure −C(=O)H, consisting of a carbonyl center (a carbon double-bonded to oxygen) with the carbon atom also bonded to hydrogen and to an R group (R= any generic alkyl or side chain). The -CHO group is the aldehyde group, also known as the formyl group. An aldehyde can also be named as alkanal.
Naming rules for aldehydes:
- The root name is based on the longest chain including the carbonyl group.
- It is not necessary to indicate the position of the -CHO group because this group will be at the end of the parent chain and its carbon is automatically assigned as C-1.
- The name is formed by changing the suffix -ane of the parent alkane to -anal.
- In other cases, such as when a -CHO group is attached to a ring, the suffix -carbaldehyde may be used.
- The term formyl- is used as prefix.
- When both double bonds and carbonyl groups are present, the -en ( not -ene) suffix follows the parent chain directly and the -al suffix follows the -en suffix.
- The location of the double bond is indicated before the root name as before, and the -al suffix follows the -en suffix directly.
- If there are more than one -CHO group, the suffix is expanded to include a prefix that indicates the number of -CHO groups present ( -edial )-
- There should not be more than 2 of these groups on the parent chain as they must occur at the ends.
- The formyl group takes precedence after nitriles and amides, but over ketones and alcohols, in the naming and numbering of the root chain. For comparison with other groups, see table for functional groups priority.
- If replacing the aldehyde group with a carboxyl group (−COOH) would yield a carboxylic acid with a trivial name, the aldehyde may be named by replacing the suffix -ic acid or -oic acid in this trivial name by -aldehyde .
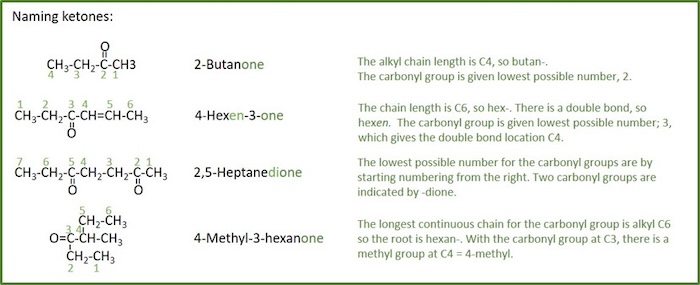
Ketones
Ketone: An organic compound with the structure R-C(=O)-R', where R and R' can be a variety of carbon-containing substituents. Ketones are also called alkanones.
Naming rules for ketones:
- The root name is based on the longest chain including the carbonyl group.
- The chain is numbered so as to give the ketone carbonyl the lowest possible number.
- Ketones are named by replacing the alkane suffix -ane with - anone .
- Ketone as substituent has the prefix oxo-
- If there is more than one carbonyl group (C=O), the suffix is expanded to include a prefix that indicates the number of carbonyl groups present ( - dione , - trione , etc.).
- The position of the carbonyl group on the parent chain is indicated by placing the number corresponding to the location on the parent chain directly in front of the base name (same as alkenes).
- The carbonyl group takes precedence over alkyl groups and halogen substituents, as well as double bonds, in the numbering of the parent chain.
- When both double bonds and carbonyl groups are present, the -en suffix follows the parent chain directly and the -one suffix follows the -en suffix (the e is left off, -en instead of -ene).
- The location of double bonds are indicated before the parent name, and the location of the carbonyl group is indicated between the -en and -one suffixes.
- If there is a choice in numbering not previously covered, the parent chain is numbered to give the substituents the lowest number at the first point of difference .
Thiones
Thione: An organic compound with the structure R-C(=S)-R', where R and R' can be a variety of carbon-containing substituents. Thiones are also called thioketones or thiocarbonyls.
Naming rules for thiones:
- The root name is based on the longest chain including the thiocarbonyl group.
- The chain is numbered so as to give the thiocarbonyl the lowest possible number.
- The suffix -thione is used to signify the presence of =S at a non-terminal carbon atom.
- Thioketone as substituent has the prefix thioxo- .
- If there is more than one thiocarbonyl group (C=S), the suffix is expanded to include a prefix that indicates the number of thiocarbonyl groups present ( - dithione , - trithione , etc.).
- The position of the thiocarbonyl group on the parent chain is indicated by placing the number corresponding to the location on the parent chain directly in front of the base name (same as alkenes).
- The thiocarbonyl group takes precedence over alkyl groups and halogen substituents, as well as double bonds, in the numbering of the parent chain. For comparison of priority with other groups, see table for functional groups priority.
- When both double bonds and thiocarbonyl groups are present, the -en suffix follows the parent chain directly and the -thione suffix follows the -en suffix (the e is left off, -en instead of -ene).
- The location of double bonds are indicated before the parent name, and the location of the thiocarbonyl group is indicated between the -en and -thione suffixes.
- If there is a choice in numbering not previously covered, the parent chain is numbered to give the substituents the lowest number at the first point of difference .
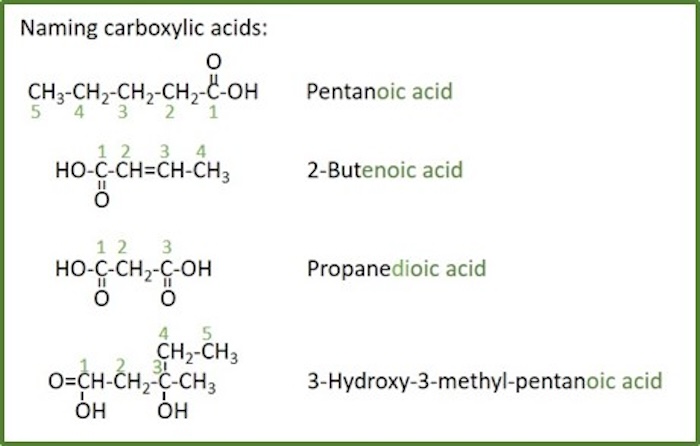
Carboxylic acids
Carboxylic acid: an organic compound that contains a carboxyl group (-C(=O)OH). The general formula of a carboxylic acid is R–COOH, with R referring to the rest of the molecule. There should not be more than 2 of carboxyl groups on the root chain as they must occur at the ends.
Alkanoic acid: A carboxylic acid where the R is an alkyl.
Branched alkanoic acid: A carboxylic acid where the R is a branched alkyl.
Dioic acid: A carboxylic acid with two acid groups, -COOH.
Benzoic acid: A carboxylic acid where the acid group is substituted to one carbon of a benzene ring.
Hydroxy acid: An carboxylic acid containing an additional hydroxyl group.
Naming rules for carboxylic acids:
- Carboxylic acids are named by counting the number of carbons in the longest continuous chain including the carboxyl group and by replacing the suffix -ane of the corresponding alkane with -anoic acid .
- If there are two -COOH groups, the suffix is expanded to include a prefix that indicates the number of -COOH groups present ( - anedioic acid ).
- It is not necessary to indicate the position of the -COOH group because this group will be at the end of the root chain and its carbon is automatically assigned as number 1.
- The carboxyl group takes precedence over all other groups in the numbering of the root chain. For comparison of priority with other groups, see table for functional groups priority.
- If the carboxyl group is attached to a ring the parent ring is named and the suffix -carboxylic acid is added.
- When both double bonds and carboxyl groups are present, the -en suffix follows the root chain directly and the -oic acid suffix follows the -en suffix (the e is left off, -en instead of -ene ).
- The location of the double bonds are indicated before the root name as before, and the -oic acid suffix follows the -en suffix directly.
- If there is a choice in numbering not previously covered, the root chain is numbered to give the substituents the lowest number at the first point of difference .
Esters
Ester: An e ster is a compound containing an oxycarbonyl group , with the generalformula R-C(=O)O-R'. It can be derived from the carboxylic acid RCO2H and the alcohol R'OH.
The easiest way to deal with naming esters is to recognise the carboxylic acid and the alcohol that they can be prepared from:
- The first component of an ester name, the alkyl is derived from the alcohol, R'-OH portion of the structure.
- The second component of an ester name, the -oate is derived from the carboxylic acid, R-CO2H portion of the structure.
- Alcohol component
- The root name is based on the longest chain containing the -OH group.
- The chain is numbered so as to give the -OH the lowest possible number.
- Carboxylic acid component
- The root name is based on the longest chain including the carbonyl group.
- Since the carboxylic acid group is at the end of the chain, it must be C1.
- The ester suffix for the acid component is appended after the hydrocarbon suffix minus the "e" : e.g. -ane + -oate = -anoate.
- The complete ester name is the alkyl alkanoate
- The oxycarbonyl group takes precedence after carboxylic acids, but over acyl halides and amides, in the naming and numbering of the root chain. For comparison with other groups, see table for functional groups priority.

Acyl halides
Acyl halide : An organic compound derived from a carboxylic acid by replacing a hydroxyl group with a halide (-Cl, -Br, -I, -F) group. The compound contains a –C(=O)-X functional group, which consists of a carbonyl group singly bonded to a halogen atom. The general formula for such an acyl halide can be written R-COX, where R may be an alkyl group, CO is the carbonyl group, and X represents the halide. Acyl halides is also called acid halide s.
The nomenclature of acid halides starts with the name of the corresponding carboxylic acid:
- The root name is based on the longest chain including the carbonyl group of the acyl group.
- The substituent suffix is - oyl halide
- The –ic acid ending is removed and replaced with the ending - yl followed by the name of the halogen with an –ide ending. This is true for both common and IUPAC nomenclature.
- The carbonyl carbon is given the location number 1. It is not necessary to include the location number in the name because it is assumed that the functional group will be on the end of the root chain.
- As substituents they have the priority before amides and nitriles, but after esters. For comparison of priority with other groups, see table for functional groups priority.
- Acyl halide as substituent has the prefix halo carbonyl- (where halo can be chloro, bromo, iodo or fluoro)
- If more than one functional group, this is indicated by using a numbering prefix (-edi-) directly before the -oyl prefix and before the halide.

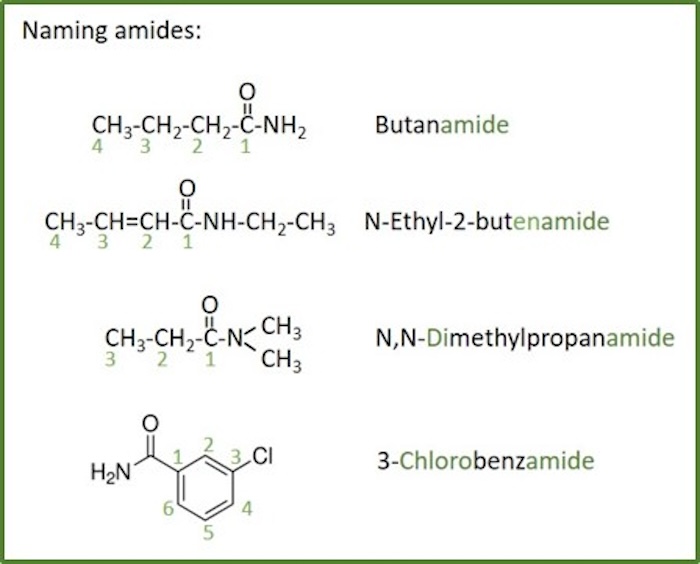
Amides
Amide: Amides are amine derivatives of carboxylic acids, by replacing a hydroxyl group with an amin group. The general formula R- C(=O)N (R',R''), where C(=O) is the carbonyl group, R may be a substituent as an alkyl group or hydrogen connected to the carbonyl, N is a nitrogen atom connected to the carbonyl and R' and R'' represents substituents as alkyl groups or hydrogen connected to the nitrogen. Amides are also called carboxamides or organic amides .
The nomenclature of amides starts with the name of the corresponding carboxylic acid:
- Amides are named by counting the number of carbons in the longest continuous chain including the carboxyl group and by replacing the suffix -ane of the corresponding alkane with -anamide .
- When both double bonds and carboxyl groups are present, the -en suffix follows the root chain directly and the -amide suffix follows the -en suffix (the e is left off, -en instead of -ene)
- Since the amide group is at the end of the chain, the C=O carbon must be C1, and i this not necessary to include the location number in the name.
- If the amide nitrogen has a substitutuent (secondary amide), this substituent are given N- as the locant.
- Tertiary amides (two substituents to the amide nitrogen) are named in the same way as secondary amides, but with two N's.
- The N- locant is listed first.
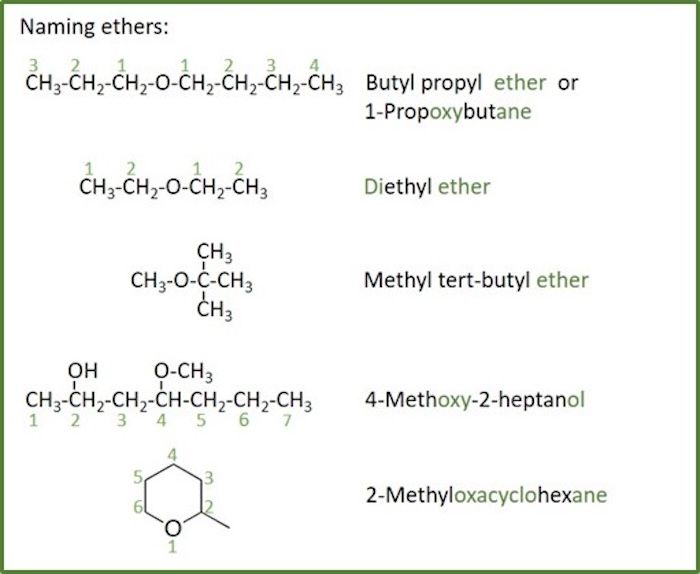
Ethers
Ether: An organic compound that have the general group R-O-R'. R and R' can be smilar or different alkyl substituents.
Naming rules for ethers:
- If both groups are simple alkyl groups, then the ether is usually named as alkyl alkyl ether.
- The two alkyl groups attached to the oxygen are put in alphabetical order with spaces between the names.
- If the two alkyl groups are the same, then it's a dialkyl ether.
- This style of naming is not used when one or more of the alkyl groups is complex or has other functional groups.
- In IUPAC, ethers are named as alkoxy- substituents with the smaller side being the substituent and the larger side being the main chain.
- As substituents they have the same priority as haloalkanes and they do not get a suffix. For comparison of priority with other groups, see table for functional groups priority.
- Ether oxygens are NOT generally part of the main chain in linear ethers.
- Cyclic ethers are named as oxacycloalkanes with the oxygen being at position 1 of the chain.
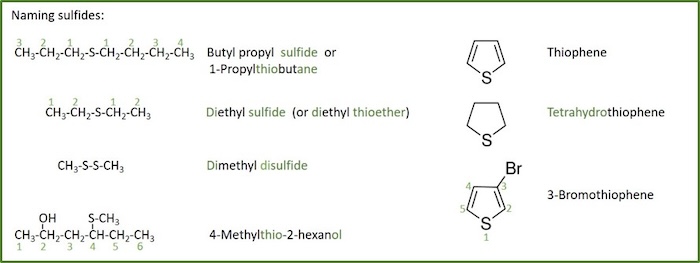
Sulfides (thioethers)
Sulfide: A sulfide is an organosulfur compound of the form R–S–R’ (where R and R’ represents an alkyl or other organic substituent).
Sulfides can be seen as a sulfur analogue to ethers (sulfur takes the place of oxygen in the alkoxy group of an ether). Sulfides are also called thioethers.
Sulfides can contain more than one S atom between the alkyl groups. They are then called disulfides (R-S-S-R') and trisulfides (R-S-S-S-R') etc.
Sulfides are named in much the same way as their oxygen cousins, and the rules for naming of sulfides (thioethers) are quite similar:
- If both groups are simple alkyl groups, then the sulfide is usually named as alkyl alkyl sulfide.
- The alkyl groups are listed in alphabetical order.
- If the two alkyl groups are the same, then it's a dialkyl sulfide (or alkyl thioether ) .
- This style of naming is not used when one or more of the alkyl groups is complex or has other functional groups.
- If one of the groups is more complex, then the sulfide group is usually treated as an alkylthio- ( i.e. R-S-) substituent.
- The more complex group ( i.e . longer chain, more branched, other substituents) defines the root.
- If both groups are complex then the sulfide (thioether) can be named using -thio
- As substituents they have priority above haloalkanes and nitrocompounds and they get the suffix sulfide . For comparison of priority with other groups, see table for functional groups priority.
- Sulfide sulfurs are NOT generally part of the main chain in linear sulfides.
- 5-membered rings with a S in the ring, are named with base in the aromatic thiophene .
- If the thiophene is fully or partially saurated, this is indicated by adding a prefix telling how many hydrogen is added to the thiophene.
- Numbering cyclic sulfides starts at the S atom, then going towards the higest priority substituent.
- Disulfides, trisulfides, etc, and polysulfides are named analogously to sulfides, except that di- , tri- , or poly-sulfide replaces sulfide , and that di- , tri- , or poly-thio- replaces thio- .
Nitros
Nitro compound: An organic compound that contain one or more nitro functional groups (−NO2), with the general formula R-NO2. R can be an alkane, alkene or other type of organic compound.
Naming rules for nitros:
- The root name is based on the longest chain containing the nitro group.
- This root give the alkane part of the name.
- The nitro group is defined as a substituent, with the prefix nitro-.
- If the nitro group is the only substituent, the chain is numbered so as to give the nitro group the lowest possible number.
- When there is a doble bond present together with the halide the double bond is given the preference (priority) in the numbering.
- The nitros have the lowest priority of the functional groups. Therefor, there are no suffixes to be used for the nitros. For comparison of priority with other groups, see table for functional groups priority.
- When there are more than one nitro group substituted to the alkyl, the number of nitro groups is indicated with prefixes di-, tri- etc. to the - nitro- name.
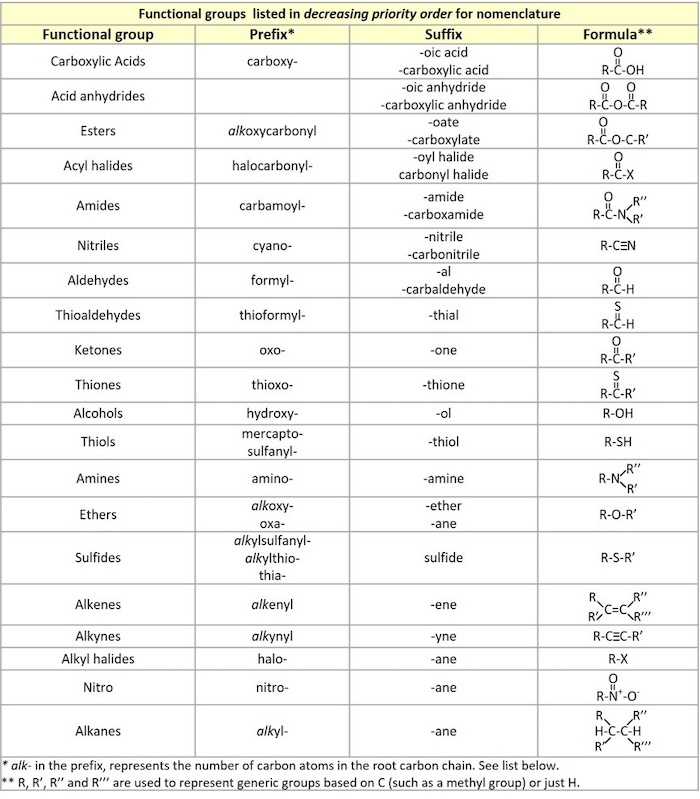
Functional groups priority
In organic chemistry, functional groups are specific groups of atoms or bonds within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of. However, its relative reactivity can be modified by other functional groups nearby. The atoms of functional groups are linked to each other and to the rest of the molecule by covalent bonds.
Combining the names of functional groups with the names of the parent alkanes generates what is termed a systematic nomenclature for naming organic compounds.
- When compounds contain more than one functional group, the order of precedence determines which groups are named with prefix or suffix forms.
- The highest-precedence group takes the suffix, with all others taking the prefix form. However, double and triple bonds only take suffix form (-en and -yn) and are used with other suffixes.
- Prefixed substituents are ordered alphabetically (excluding any modifiers such as di-, tri-, etc.), e.g. chlorofluoromethane, not fluorochloromethane.
- If there are multiple functional groups of the same type, either prefixed or suffixed, the position numbers are ordered numerically.
This priority order is important in nomenclature as the higher priority group is the principal functional group and it is typically numbered such that it has the lowest number (the lowest locant ).
Note that aromatic systems (arenes) such as a benzene ring should also be thought of as a functional group, but they don't fit into the priority order list shown below.
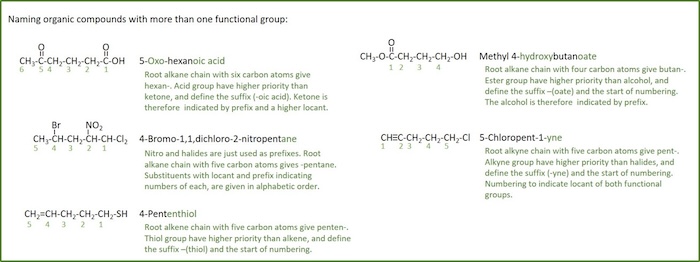
Examples of usage of the prioritizing rules in naming of organic compounds with more than one functional group are shown in figure below:
Acyl halides
Alcohols
Aldehydes
Alkanes
Alkenes
Alkynes
Amides
Amines
Aromatics
Carboxylic acids
Esters
Ethers
Functional groups priority
General naming principles
Greek numbers used as prefixes
Haloalkanes (alkylhalides)
Ketones
Nitriles
Nitros
Sulfides (thioethers)
Th iols (mercaptans)
Thiones (thioketones)




