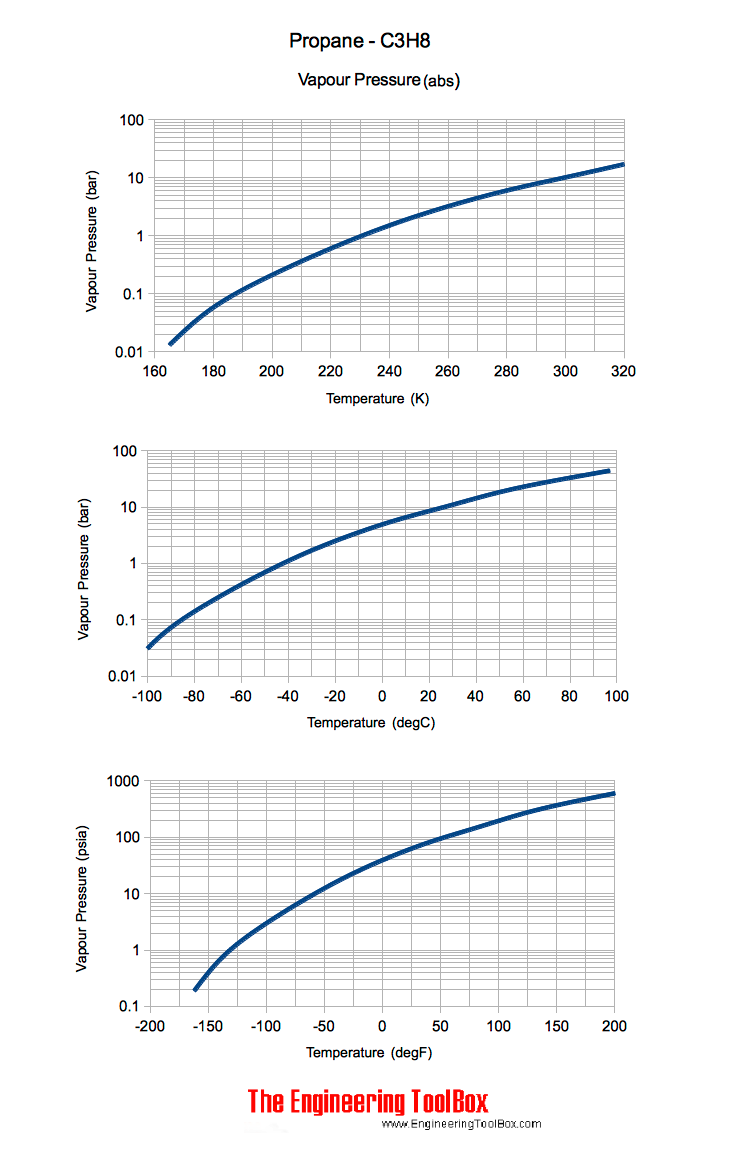
Propane - Vapor Pressure vs. Temperature
Vapor pressure vs. temperature.
The vapor pressure of propane (C3H8) depends on the temperature. Vapor pressure of 100% propane:
Note! The metric chart indicates gauge pressure. The Imperial chart indicates absolute pressure . Imperial gauge pressure can be calculated as
psig = psia - 14.7 (psi)
See also other properties of Propane at varying temperature and pressure : Density and specific weight , Dynamic and Kinematic Viscosity , Prandtl number , Specific heat (heat capacity) , Thermal conductivity and Thermal diffusivity , and Thermophysical properties at standard conditions .
Related Topics
-
Combustion
Combustion processes and their efficiency. Boiler house and chimney topics. Properties of fuels like oil, gas, coal and wood and more. Safety valves and tanks.
Related Documents
-
Adiabatic Flame Temperatures
Adiabatic flame temperatures for hydrogen, methane, propane and octane - in Kelvin. -
Gases - Explosion and Flammability Concentration Limits
Flame and explosion limits for gases like propane, methane, butane, acetylene and more. -
Hydrocarbons - Vapor Pressures
Vapor pressure vs. temperature for propane, n-butane, n-heptane and n-pentane hydrocarbons. -
Liquids - Densities
Densities of common liquids like acetone, beer, oil, water and more. -
Liquids - Vapor Pressures
Vapor and saturation pressure for some common liquids. -
LPG - Gas Properties
Liquefied Petroleum - LP - gas properties. -
LPG Pipes - Pressure Loss vs. Gas Flow
Resistance and pressure loss in liquid LPG pipes. -
LPG Tanks - Relief Valves Capacities
Required capacities of relief valves on LPG vaporizers and tanks. -
Propane - Density and Specific Weight vs. Temperature and Pressure
Online calculator, figures and tables showing density and specific weight of propane, C3H8, at temperatures ranging from -187 to 725 °C (-305 to 1300 °F) at atmospheric and higher pressure - Imperial and SI Units. -
Propane - Dynamic and Kinematic Viscosity vs. Temperature and Pressure
Online calculators, figures and tables showing dynamic and kinematic viscosity of liquid and gaseous propane at varying temperarure and pressure, SI and Imperial units. -
Propane - Latent Heat of Vaporization vs. Temperature
Latent heat with vaporized propane. -
Propane - Prandtl Number vs. Temperature and Pressure
Figures and tables with Prandtl Number of liquid and gaseous propane at varying temperarure and pressure, SI and Imperial units. -
Propane - Thermal Conductivity vs. Temperature and Pressure
Online calculator, figures and tables showing thermal conductivity of liquid and gaseous propane at varying temperarure and pressure, SI and Imperial units. -
Propane - Thermal Diffusivity vs. Temperature and Pressure
Figures and tables showing thermal diffusivity of liquid and gaseous propane at varying temperarure and pressure, SI and Imperial units. -
Propane - Thermophysical properties
Chemical, physical and thermal properties of propane gas - C3H8. -
Propane Air Mixture
Energy content and specific gravity of propane air mixtures. -
Propane Butane Mixture - Evaporation Pressure
Evaporation pressure of propane butane mixture vs. temperature. -
Propane Gas - Sizing of Pipe Lines
Sizing low pressure propane gas pipe lines - Metric units. -
Propane Gas Piping - Capacity vs. Size
Sizing of propane gas pipe lines with pressures above 5 psig (35 kPa).