Nitrogen - Thermal Diffusivity vs. Temperature and Pressure
Figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, SI and Imperial units.
Thermal diffusivity is the rate of transfer of heat of a material from the hot side to the cold side - a measure of how quickly a material can absorb heat from its surroundings . It can be calculated by taking the thermal conductivity divided by density and specific heat capacity at constant pressure.
Thermal diffusivity has the SI derived unit of (m²/s) , and is usually denoted α but a, κ, K, and D are also used. The formula is:
α = k / (ρ CP) (1)
where
k = is thermal conductivity (W/(m·K))
ρ = density (kg/m³)
CP = specific heat capacity (J/(kg·K))
Below, thermal diffusivity of nitrogen at varying temperatures and 1, 10, 50 and 100 bara (14.5, 145, 725 and 1450 psia) are given in figures and tables.
See also other properties of Nitrogen at varying temperature and pressure : Density and specific weight, Dynamic and kinematic viscosity, Prandtl number, Specific heat (Heat capacity) and Thermal conductivity, and thermophysical properties at standard conditions,
as well as thermal diffusivity of air, propane and water .
Thermal diffusivity of nitrogen at 1, 10, 50 and 100 bara (14.5, 145, 725 and 1450 psia), and varying temperature given as °C or °F:
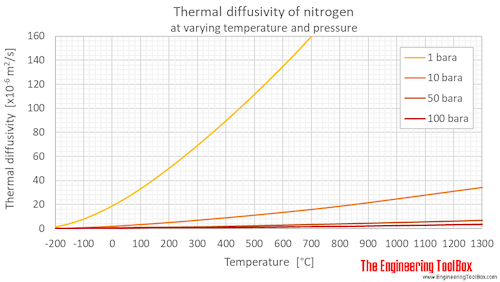
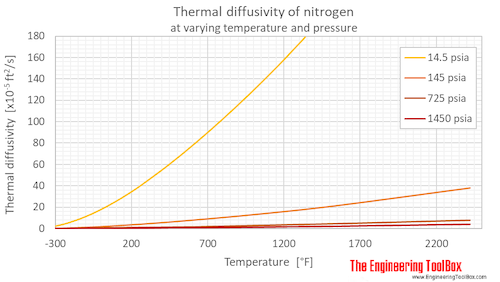
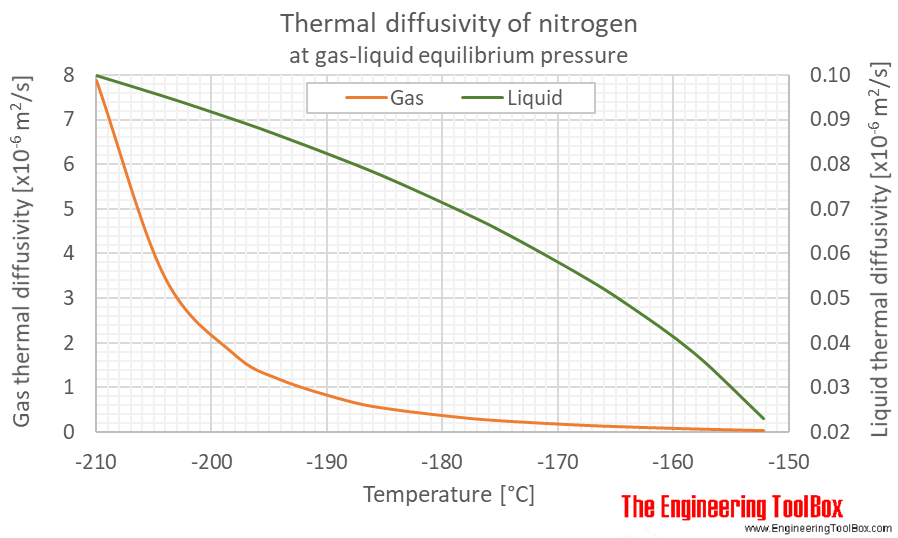
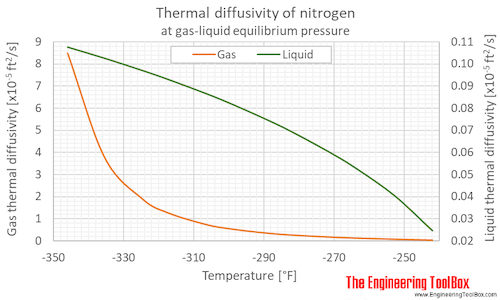
Thermal diffusivity of nitrogen at gas-liquid equilibrium pressure and varying temperature given as °C or °F:
Thermal diffusivity of gaseous nitrogen at atmospheric pressure and given temperature, SI and Imperial units:
| Temperature | Thermal diffusivity | Temperature | Thermal diffusivity | |||||
|---|---|---|---|---|---|---|---|---|
| (K) | (°C) | (×10-6 m2/s) | (m2/h) | (K) | (°F) | (×10-5ft2/s) | ( ft2/h) | |
| 78 | -195 | 1.489 | 0.00536 | 78 | -320 | 1.577 | 0.05679 | |
| 98 | -175 | 2.447 | 0.00881 | 89 | -300 | 2.118 | 0.07626 | |
| 123 | -150 | 3.934 | 0.01416 | 116 | -250 | 3.775 | 0.1359 | |
| 148 | -125 | 5.727 | 0.02062 | 144 | -200 | 5.843 | 0.2103 | |
| 173 | -100 | 7.807 | 0.02811 | 172 | -150 | 8.298 | 0.2987 | |
| 198 | -75 | 10.16 | 0.03658 | 200 | -100 | 11.11 | 0.4001 | |
| 223 | -50 | 12.77 | 0.04597 | 228 | -50 | 14.27 | 0.5137 | |
| 231 | -42 | 13.65 | 0.04916 | 231 | -44 | 14.67 | 0.5281 | |
| 248 | -25 | 15.62 | 0.05622 | 244 | -20 | 16.32 | 0.5874 | |
| 263 | -10 | 17.44 | 0.06277 | 255 | 0 | 17.74 | 0.6387 | |
| 268 | -5 | 18.06 | 0.06501 | 266 | 20 | 19.21 | 0.6917 | |
| 278 | 5 | 19.33 | 0.06960 | 278 | 40 | 20.73 | 0.7464 | |
| 283 | 10 | 19.98 | 0.07193 | 283 | 50 | 21.51 | 0.7743 | |
| 293 | 20 | 21.30 | 0.07670 | 294 | 70 | 23.09 | 0.8313 | |
| 298 | 25 | 21.98 | 0.07912 | 300 | 80 | 23.90 | 0.8604 | |
| 303 | 30 | 22.66 | 0.08158 | 305 | 90 | 24.72 | 0.8899 | |
| 323 | 50 | 25.46 | 0.09167 | 311 | 100 | 25.55 | 0.9198 | |
| 348 | 75 | 29.14 | 0.1049 | 339 | 150 | 29.85 | 1.074 | |
| 373 | 100 | 32.99 | 0.1187 | 366 | 200 | 34.38 | 1.238 | |
| 398 | 125 | 37.00 | 0.1332 | 394 | 250 | 39.14 | 1.409 | |
| 423 | 150 | 41.16 | 0.1482 | 422 | 300 | 44.10 | 1.588 | |
| 448 | 175 | 45.47 | 0.1637 | 478 | 400 | 54.59 | 1.965 | |
| 473 | 200 | 49.91 | 0.1797 | 533 | 500 | 65.75 | 2.367 | |
| 573 | 300 | 68.88 | 0.2480 | 644 | 700 | 89.74 | 3.231 | |
| 623 | 350 | 78.99 | 0.2844 | 700 | 800 | 102.5 | 3.689 | |
| 673 | 400 | 89.46 | 0.3221 | 755 | 900 | 115.6 | 4.163 | |
| 773 | 500 | 111.4 | 0.4011 | 811 | 1000 | 129.2 | 4.652 | |
Thermal diffusivity of nitrogen at given temperature and pressure, SI and Imperial units:
| State | Temperature | Pressure | Thermal diffusivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (K) | (°C) | (°F) | (bara) | (psia) | ( ×10-6 m2/s) | (m2/h) | (×10-5ft2/s) | ( ft2/h) | |
| Liquid at equilibrium |
63.15 | -210.0 | -346.0 | 0.125 | 1.82 | 0.09987 | 3.60E-04 | 0.1075 | 0.00387 |
| 69 | -204 | -335 | 0.332 | 4.82 | 0.09523 | 3.43E-04 | 0.1025 | 0.00369 | |
| 75 | -198 | -325 | 0.760 | 11.0 | 0.09011 | 3.24E-04 | 0.09699 | 0.00349 | |
| 79 | -194 | -317 | 1.22 | 17.8 | 0.08644 | 3.11E-04 | 0.09304 | 0.00335 | |
| 85 | -188 | -307 | 2.29 | 33.2 | 0.08053 | 2.90E-04 | 0.08668 | 0.00312 | |
| 89 | -184 | -299 | 3.31 | 47.9 | 0.07624 | 2.74E-04 | 0.08206 | 0.00295 | |
| 95 | -178 | -289 | 5.41 | 78.4 | 0.06918 | 2.49E-04 | 0.07447 | 0.00268 | |
| 99 | -174 | -281 | 7.26 | 105 | 0.06401 | 2.30E-04 | 0.06890 | 0.00248 | |
| 105 | -168 | -271 | 10.8 | 157 | 0.05539 | 1.99E-04 | 0.05962 | 0.00215 | |
| 109 | -164 | -263 | 13.8 | 201 | 0.04891 | 1.76E-04 | 0.05265 | 0.00190 | |
| 115 | -158 | -253 | 19.4 | 281 | 0.03766 | 1.36E-04 | 0.04054 | 0.00146 | |
| 121 | -152 | -242 | 26.4 | 383 | 0.02302 | 8.29E-05 | 0.02478 | 0.00089 | |
| Gas at equilibrium |
63.15 | -210.0 | -346.0 | 0.125 | 1.82 | 7.877 | 0.02836 | 8.478 | 0.30523 |
| 69 | -204 | -335 | 0.332 | 4.82 | 3.506 | 0.01262 | 3.774 | 0.13585 | |
| 75 | -198 | -325 | 0.760 | 11.0 | 1.763 | 0.00635 | 1.897 | 0.06830 | |
| 79 | -194 | -317 | 1.22 | 17.8 | 1.182 | 0.00426 | 1.273 | 0.04582 | |
| 85 | -188 | -307 | 2.29 | 33.2 | 0.6937 | 0.00250 | 0.7467 | 0.02688 | |
| 89 | -184 | -299 | 3.31 | 47.9 | 0.5022 | 0.00181 | 0.5406 | 0.01946 | |
| 95 | -178 | -289 | 5.41 | 78.4 | 0.3198 | 0.00115 | 0.3442 | 0.01239 | |
| 99 | -174 | -281 | 7.26 | 105 | 0.2398 | 8.63E-04 | 0.2581 | 0.00929 | |
| 105 | -168 | -271 | 10.8 | 157 | 0.1562 | 5.62E-04 | 0.1681 | 0.00605 | |
| 109 | -164 | -263 | 13.8 | 201 | 0.1159 | 4.17E-04 | 0.1248 | 0.00449 | |
| 115 | -158 | -253 | 19.4 | 281 | 0.06909 | 2.49E-04 | 0.07437 | 0.00268 | |
| 121 | -152 | -242 | 26.4 | 383 | 0.03166 | 1.14E-04 | 0.03407 | 0.00123 | |
| Liquid | 63.17 | -210.0 | -346.0 | 1 | 14.5 | 0.09991 | 3.60E-04 | 0.1075 | 0.00387 |
| 77.24 | -195.9 | -320.6 | 1 | 14.5 | 0.08808 | 3.17E-04 | 0.09481 | 0.00341 | |
| Gas | 77.24 | -195.9 | -320.6 | 1 | 14.5 | 1.402 | 0.00505 | 1.509 | 0.05433 |
| 80 | -193.2 | -315.7 | 1 | 14.5 | 1.529 | 0.00550 | 1.645 | 0.05923 | |
| 100 | -173.2 | -279.7 | 1 | 14.5 | 2.548 | 0.00917 | 2.743 | 0.09875 | |
| 120 | -153.2 | -243.7 | 1 | 14.5 | 3.754 | 0.01352 | 4.041 | 0.1455 | |
| 140 | -133.2 | -207.7 | 1 | 14.5 | 5.151 | 0.01854 | 5.544 | 0.1996 | |
| 160 | -113.2 | -171.7 | 1 | 14.5 | 6.721 | 0.02420 | 7.234 | 0.2604 | |
| 180 | -93.2 | -135.7 | 1 | 14.5 | 8.464 | 0.03047 | 9.110 | 0.3280 | |
| 200 | -73.2 | -99.7 | 1 | 14.5 | 10.38 | 0.03738 | 11.18 | 0.4023 | |
| 220 | -53.2 | -63.7 | 1 | 14.5 | 12.44 | 0.04478 | 13.39 | 0.4820 | |
| 240 | -33.2 | -27.7 | 1 | 14.5 | 14.67 | 0.05282 | 15.79 | 0.5685 | |
| 260 | -13.2 | 8.3 | 1 | 14.5 | 17.03 | 0.06129 | 18.33 | 0.6598 | |
| 280 | 6.9 | 44.3 | 1 | 14.5 | 19.56 | 0.07040 | 21.05 | 0.7578 | |
| 300 | 26.9 | 80.3 | 1 | 14.5 | 22.21 | 0.07997 | 23.91 | 0.8608 | |
| 320 | 46.9 | 116.3 | 1 | 14.5 | 24.96 | 0.08987 | 26.87 | 0.9673 | |
| 340 | 66.9 | 152.3 | 1 | 14.5 | 27.88 | 0.1004 | 30.01 | 1.080 | |
| 360 | 86.9 | 188.3 | 1 | 14.5 | 30.89 | 0.1112 | 33.25 | 1.197 | |
| 400 | 126.9 | 260.3 | 1 | 14.5 | 37.28 | 0.1342 | 40.13 | 1.445 | |
| 500 | 226.9 | 440.3 | 1 | 14.5 | 54.88 | 0.1976 | 59.08 | 2.127 | |
| 600 | 326.9 | 620.3 | 1 | 14.5 | 74.31 | 0.2675 | 79.99 | 2.880 | |
| 700 | 426.9 | 800.3 | 1 | 14.5 | 95.24 | 0.3429 | 102.5 | 3.691 | |
| 800 | 526.9 | 980.3 | 1 | 14.5 | 117.5 | 0.4231 | 126.5 | 4.554 | |
| 900 | 626.9 | 1160.3 | 1 | 14.5 | 141.1 | 0.5081 | 151.9 | 5.469 | |
| 1000 | 726.9 | 1340.3 | 1 | 14.5 | 166.3 | 0.5986 | 179.0 | 6.444 | |
| Liquid | 63.37 | -210 | -346 | 10 | 145 | 0.1003 | 3.61E-04 | 0.1079 | 0.00388 |
| 80 | -193 | -316 | 10 | 145 | 0.08632 | 3.11E-04 | 0.09292 | 0.00334 | |
| 100 | -173 | -280 | 10 | 145 | 0.06318 | 2.27E-04 | 0.06800 | 0.00245 | |
| 103.8 | -169.4 | -272.9 | 10 | 145 | 0.05728 | 2.06E-04 | 0.06166 | 0.00222 | |
| Gas | 103.8 | -169.4 | -272.9 | 10 | 145 | 0.1710 | 6.15E-04 | 0.1840 | 0.00662 |
| 140.0 | -133.2 | -207.7 | 10 | 145 | 0.4573 | 0.00165 | 0.4922 | 0.01772 | |
| 160 | -113 | -172 | 10 | 145 | 0.6261 | 0.00225 | 0.6739 | 0.02426 | |
| 180 | -93 | -136 | 10 | 145 | 0.8087 | 0.00291 | 0.8704 | 0.03134 | |
| 200 | -73.2 | -99.7 | 10 | 145 | 1.007 | 0.00363 | 1.084 | 0.03903 | |
| 240 | -33.2 | -27.7 | 10 | 145 | 1.448 | 0.00521 | 1.559 | 0.05613 | |
| 300 | 26.9 | 80.3 | 10 | 145 | 2.218 | 0.00798 | 2.387 | 0.08593 | |
| 400 | 127 | 260 | 10 | 145 | 3.730 | 0.01343 | 4.015 | 0.1445 | |
| 500 | 227 | 440 | 10 | 145 | 5.489 | 0.01976 | 5.908 | 0.2127 | |
| 600 | 327 | 620 | 10 | 145 | 7.464 | 0.02687 | 8.035 | 0.2892 | |
| 800 | 527 | 980 | 10 | 145 | 11.76 | 0.04232 | 12.65 | 0.4555 | |
| 1100 | 827 | 1520 | 10 | 145 | 19.35 | 0.06966 | 20.83 | 0.7498 | |
| 1600 | 1327 | 2420 | 10 | 145 | 35.06 | 0.1262 | 37.74 | 1.359 | |
| Liquid | 100 | -173 | -280 | 50 | 725 | 0.07101 | 2.56E-04 | 0.07643 | 0.00275 |
| Supercritical phase |
600 | 327 | 620 | 50 | 725 | 1.529 | 0.00550 | 1.646 | 0.05925 |
| 1100 | 827 | 1520 | 50 | 725 | 3.933 | 0.01416 | 4.233 | 0.1524 | |
| 1600 | 1327 | 2420 | 50 | 725 | 7.093 | 0.02553 | 7.635 | 0.2748 | |
| Liquid | 65.32 | -208 | -342 | 100 | 1450 | 0.1035 | 3.73E-04 | 0.1114 | 0.00401 |
| 80 | -193 | -316 | 100 | 1450 | 0.09376 | 3.38E-04 | 0.1009 | 0.00363 | |
| 100 | -173 | -280 | 100 | 1450 | 0.07813 | 2.81E-04 | 0.084 | 0.00303 | |
| Supercritical phase |
200 | -73.2 | -99.7 | 100 | 1450 | 0.09050 | 3.26E-04 | 0.097 | 0.00351 |
| 300 | 26.9 | 80.3 | 100 | 1450 | 0.2333 | 8.40E-04 | 0.2511 | 0.00904 | |
| 400 | 127 | 260 | 100 | 1450 | 0.4001 | 0.00144 | 0.4307 | 0.01550 | |
| 500 | 227 | 440 | 100 | 1450 | 0.5882 | 0.00212 | 0.6331 | 0.02279 | |
| 600 | 327 | 620 | 100 | 1450 | 0.7922 | 0.00285 | 0.8527 | 0.03070 | |
| 1100 | 827 | 1520 | 100 | 1450 | 2.008 | 0.00723 | 2.162 | 0.07782 | |
| 1600 | 1327 | 2420 | 100 | 1450 | 3.598 | 0.01295 | 3.873 | 0.1394 | |
Unit conversion thermal diffusivity:
square feet/second (ft2/s), square feet/hour (ft2/h), square meter/hour (m2/h), square meter/second (m2/s)
- 1 ft2/h = 2.7778×10-4 ft2/s = 0.09290 m2/h = 2.581×10-5m2/s
- 1 ft2/s = 3600 ft2/h = 334.45 m2/h = 0.09290 m2/s
- 1 m2/h = 2.7778×10-4 m2/s = 10.7639 ft2/h = 0.002990 ft2/s
- 1 m2/s = 3600 m2/h = 38750.1 ft2/h = 10.7639 ft2/s



