Domestic Water Supply - Lime Deposits
Lime deposited vs. temperature and water consumption.
Lime deposited in water supply systems - based on 170 mg/liter dissolved calcium and magnesium (hard water):
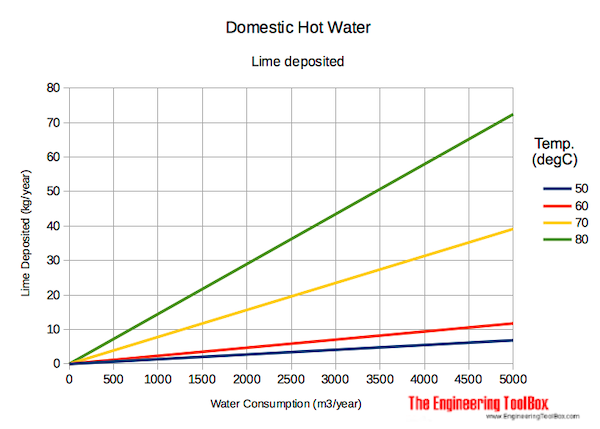
Lime deposits increase with temperature due to less calcium carbonate solubility at higher temperatures.
Example - Deposits from Consumption of Hard Water
The deposits from hard water with 170 mg dissolved calcium and magnesium per liter, temperature 60 oC and water consumption 2000 m3/year - can from the diagram above be estimated to aprox.
5 kg/year



