Air - Humidity Ratio
The mass of water vapor present in moist air - to the mass of dry air.
The humidity ratio of moist air can be expressed with
- the mass of water vapor in the humid air - to the mass of dry air, or by
- the partial pressure of vapor in the air - to the partial pressure of the dry air
Humidity Ratio by Mass
Humidity ratio by mass can be expressed as
- the ratio between the actual mass of water vapor present in moist air - to the mass of the dry air
Humidity ratio is normally expressed in kilograms (or pounds) of water vapor per kilogram (or pound) of dry air.
Humidity ratio expressed by mass:
x = mw / ma (1)
where
x = humidity ratio (kgwater/kgdry_air, lbwater/lbdry_air)
mw = mass of water vapor (kg, lb)
ma = mass of dry air (kg, lb)
Humidity Ratio by Vapor Partial Pressure
Based on the Ideal Gas Law the humidity ratio can be expressed as
x = 0.62198 pw / (pa - pw) (2)
where
pw = partial pressure of water vapor in moist air (Pa, psi)
pa = atmospheric pressure of moist air (Pa, psi)
The maximum amount of water vapor in the air is achieved when pw =pws the saturation pressure of water vapor at the actual temperature. (2) can be modified to:
xs = 0.62198 pws / (pa - pws) (3)
where
xs = maximum saturation humidity ratio of air (kgwater/kgair, lbwater/lbdry_air)
The water vapor pressure is small regarding to the atmospheric pressure and the relation between the humidity ratio and the saturation pressure is almost linear.
Note! - be careful with these equations at higher temperatures - as indicated in Temperature and Moisture Holding Capacity of Air.
Maximum specific humidity at some common temperatures:
| Temperature (oC) | Water Vapor Saturation Pressure (Pa) | Maximum Saturation Humidity Ratio - x - (kgw/kga) |
|---|---|---|
| 0 | 609.9 | 0.003767 |
| 5 | 870 | 0.005387 |
| 10 | 1225 | 0.007612 |
| 15 | 1701 | 0.01062 |
| 20 | 2333 | 0.014659 |
| 25 | 3130 | 0.019826 |
| 30 | 4234 | 0.027125 |
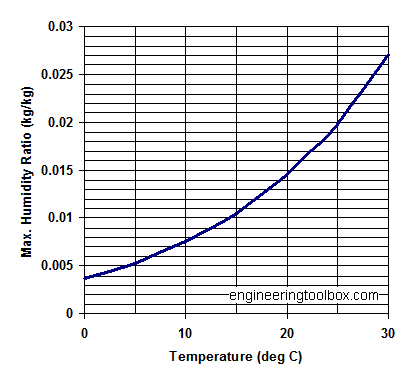
Note that the saturation pressure of water vapor, - and the maximum humidity ratio, increases dramatically with air temperature. This important for the capacity of drying processes.
Example - Humidity Ratio of Moist Air
The humidity ratio for saturated moist air at 20oC with water vapor partial pressure 2333 Pa at atmospheric pressure of 101325 Pa (1013 mbar, 760 mmHg) can be calculated as:
x = 0.62198 (2333 Pa) / ((101325 Pa) - (2333 Pa))
= 0.0147 (kg/kg)
= 14.7 (g/kg)
Related Topics
-
Air Psychrometrics
Moist and humid air calculations. Psychrometric charts and Mollier diagrams. Air-condition systems temperatures, absolute and relative humidities and moisture content in air.
Related Documents
-
Air - Absolute Humidity
Absolute humidity is the actual mass of water vapor present in the air water vapor mixture. -
Air - Composition and Molecular Weight
Dry air is a mechanical mixture of nitrogen, oxygen, argon and several other gases in minor amounts. -
Air - Density vs. Pressure and Temperatures
Air density at pressure ranging 1 to 10 000 bara (14.5 - 145000 psi) and constant selected temperatures. -
Air - Density, Specific Weight and Thermal Expansion Coefficient vs. Temperature and Pressure
Online calculator, figures and tables showing density, specific weight and thermal expansion coefficients of air at temperatures ranging -100 to 1600 °C (-140 to 2900 °F) at atmospheric and higher pressure - Imperial and SI Units. -
Air - Diffusion Coefficients of Gases in Excess of Air
Diffusion coefficients (D12) for gases in large excess of air at temperatures ranging 0 - 400 °C. -
Air - Drying Force
The drying force of air depends on the air moisture holding capacity and the water surface to air evaporation capacity. -
Air - Dynamic and Kinematic Viscosity
Online calculator, figures and tables with dynamic (absolute) and kinematic viscosity for air at temperatures ranging -100 to 1600°C (-150 to 2900°F) and at pressures ranging 1 to 10 000 bara (14.5 - 145000 psia) - SI and Imperial Units. -
Air - Humidifying with Steam - Imperial Units
Estimate the amount of steam required (lb/h in 100 cfm) in humid air. -
Air - Humidifying with Steam, SI units
Using steam to humidify air. -
Air - Humidity Measurement from Dry and Wet Bulb Temperature
Relative humidity in moist air can estimated by measuring the dry and wet bulb temperature. -
Air - Maximum Moisture Carrying Capacity
Maximum water content in humid air vs. temperature. -
Air - Moisture Holding Capacity vs. Temperature
The moisture holding capacity of air increases with temperature. -
Air - Prandtl Number
Prandtl number for air vs. temperature and pressure. -
Air - Properties at Gas-Liquid Equilibrium Conditions
Properties of air change along the boiling and condensation curves (temperature and pressure between triple point and critical point conditions). An air phase diagram included. -
Air - Specific Heat vs. Pressure at Constant Temperature
Figures and tables with isobaric (Cp) and isochoric (Cv) specific heat of air at constant temperature and pressure ranging 0.01 to 10000 bara. -
Air - Thermal Conductivity vs. Temperature and Pressure
Online calculator with figures and tables showing air thermal conductivity vs. temperature and pressure. SI and imperial units. -
Air - Thermal Diffusivity vs. Temperature and Pressure
Figures and tables withdry air thermal diffusivity vs. temperarure and pressure. SI and Imperial units. -
Dehumidifiers
Classification of dehumidifiers. -
Dry Air - Thermodynamic and Physical Properties
Thermodynamic properties of dry air - specific heat, ratio of specific heats, dynamic viscosity, thermal conductivity, Prandtl number, density and kinematic viscosity at temperatures ranging 175 - 1900 K. -
Evaporation from a Water Surface
Evaporation of water from a water surface - like a swimming pool or an open tank - depends on water temperature, air temperature, air humidity and air velocity above the water surface - online calculator. -
Evaporative Cooling
Evaporative cooling tutorial. -
Great Sensible Heat Factor - GSHF
The Great Sensible Heat Factor is the ratio sensible to total heat in a cooling coil. -
Latent Heat Flow
Latent heat is the heat when supplied to or removed from air results in a change in moisture content - the temperature of the air is not changed. -
Mixing of Humid Air
The change in state wwhen mixing moist air - enthalpy, heat, temperature and specific humidity. -
Moist Air - Cooling and Dehumidifying
Cooling and dehumidifying processes of moist and humid air - sensible and latent cooling. -
Moist Air - Degree of Saturation
Humidity ratio of moist air to humidity ratio of saturated moist air. -
Moist Air - Density vs. Water Content and Temperature
Density of the mix of dry air and water vapor - moist humid air. -
Moist Air - Enthalpy
Sensible and latent heat of moist air. -
Moist Air - Psychrometric Terms
Dry and wet bulb temperature, specific volume, relative humidity, enthalpy and more. -
Moist Air - Relative Humidity
Relative humidity in moist air is the ratio of partial vapor pressure to air pressure. -
Moist Air - Specific Volume
Specific volume of moist air is defined as the total volume of humid air per mass unit of dry air -
Moist Air - Specific vs. Relative Humidity
Specific humidity of moist air vs. relative humidity, water vapor and air density. -
Moisture Content Calculation
Calculate the moisture content in products like wood on wet and dry basis. -
Recommended Relative Humidity
Recommended relative humidity level. -
Relative Humidity in Production and Process Environments
Recommended relative humidity in production and process environments - like libraries, breweries, storages and more. -
Required Air Flow to Remove Moisture
Air flow required to remove vapor production from a room. -
Saturated Salt Solutions - Controlling Air Humidity
A salt solutions can be used maintain a particular value of relative humidity. -
Water - Saturation Pressure vs. Temperature
Online calculator, figures and tables with water saturation (vapor) pressure at temperatures ranging 0 to 370 °C (32 to 700°F) - in Imperial and SI Units.




