Mixing Liquids and/or Solids - Final Temperatures
Calculate the final temperature when liquids or solids are mixed
When liquids and/or solids with different temperatures are mixed together - the final mixed temperature can be calculated as
tf = (m1 cp1 t1 + m2 cp2 t2 + .... + mn cpn tn) / (m1 cp1 + m2 cp2 + .... + mn cpn) (1)
where
tf = final mixed temperature (oC)
m = mass of substance (kg)
cp = specific heat of substance (J/kgoC)
t = temperature of substance (oC)
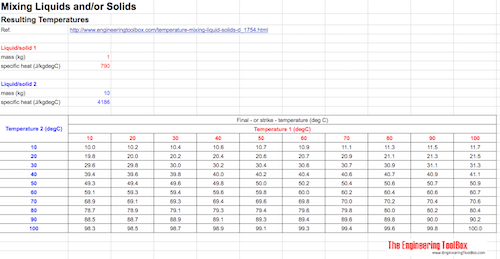
Example - a heated stone in a bucket of water
A granite stone of 1 kg (specific heat 790 J/kg.oC) is heated to 100 oC and added to a bucket of 10 kg water (specific heat 4186 J/kgoC) at 20 oC. The final temperature of the water - stone "mix" can be calculated as
tf = ((1 kg) (790 J/kgoC) (100 oC) + (10 kg) (4186 J/kgoC) (20 oC)) / ((1 kg) (790 J/kgoC) + (10 kg) (4186 J/kgoC))
= 21.5 oC
Mixed Temperature Calculator
The calculator below is based on eq. 1 and can be used to calculate the final - strike - temperature when two liquids or solids - or a liquid and a solid - is mixed together.
Mixing Liquids and/or Solids - Final Temperatures Spreadsheet Calculation
The Google Docs spreadsheet below can be used to calculate final temperature when mixing fluids or solids. You can copy or download the spreadsheet to make your own copy.