Electric Heating of Mass
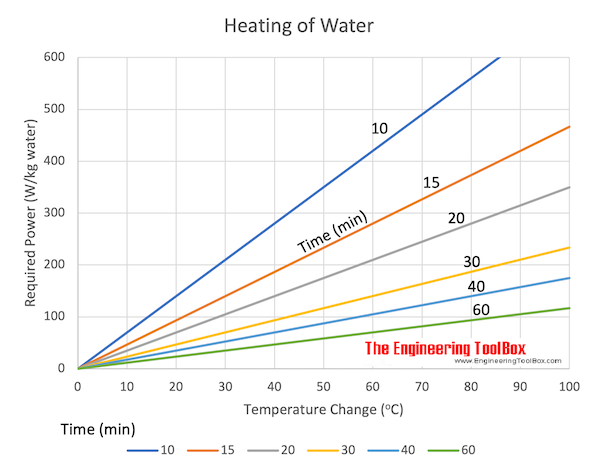
Electric heating and temperature change vs. energy supplied.
When an electric heater heats an object - the electric energy is transformed to heat by increased object temperature. The electric energy used equals the change in thermal energy and can be expressed as:
P t μ = U I t μ
= c m dT (1)
electric energy supplied = thermal energy change in object
where
P = power (watts, W)
U = potential difference (volts, V)
I = electrical current (amps, A)
t = time (sec)
μ = heating efficiency
c = specific heat of the heated substance (J/kgoC)
m = mass of the heated substance (kg)
dT = temperature differance (oC)
Example - 12 Volt Car Water Heater

The temperature change in a 12 volts and 10 amps Car Water Heater (100% efficient => μ = 1) with 0.4 kg water and 5 minutes heating period can be calculated by modifying (1) to
dT = U I t μ / c m
= (12 volts) (10 amps) (300 sec) (1) / (4200 J/kgoC) (0.4 kg)
= 21.4 oC
Note! The mass of the water heater and the heat loss from the heater during heating is neglected in the calculation. In reality the mass of the heater and the heat loss from the heater during the heating process will influence the temperature change.
Electric Heating Calculator
This calculator can be used to calculate temperature rise in an electric heated object or mass. Heat loss during heating is neglected.