Ammonia - Vapour Pressure at Gas-Liquid Equilibrium
Figures and table with ammonia saturation pressure at boiling points, SI and Imperial units.
Anhydrous ammonia , NH3 : Thermophysical properties
- a colorless non-flammable liquefied gas
- vapor is lighter than air - 0.6 compared to air 1.0
- ignition temperature 1204 oF (651 oC) (vapor concentration between 15% and 28%)
- corrodes galvanized metals, cast iron, copper, brass or copper alloys
- weight of liquid ammonia 5.15 pounds per gallon (water weight 8.33 pounds per gallon)
- boiling point liquid ammonia at atmospheric pressure -28 oF (-33.3 oC)
- liquid ammonia expands to 850 times its liquid volume at atmospheric pressure
The vapour pressure of ammonia is the pressure at which ammonia gas is in thermodynamic equilibrium with its condensed state . At higher pressures ammonia would condense . At this equilibrium condition the vapor pressure is the saturation pressure .
See also properties of Ammonia at varying temperature and pressure: Density and specific weight, Dynamic and kinematic viscosity, Prandtl Number, Specific Heat (Heat Capacity) and Thermal Conductivity, and Thermophysical properties at standard conditions.
Ammonia Temperature Pressure Diagram
Saturation (boiling pressures) of liquid ammonia at different temperatures are indicated in the figures below. An ammonia phase diagram is also included:
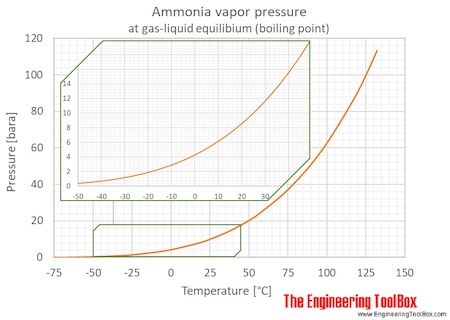
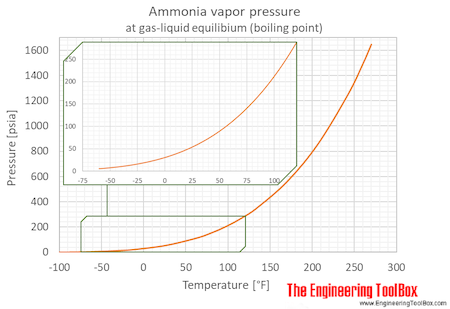

| Temperature | Pressure | Vacuum1) | |||||
|---|---|---|---|---|---|---|---|
| (K) | (°C) | (°F) | (MPa) | (bara) | (psia) | (mm Hg), (torr) | (in Hg) |
| 222 | -51.1 | -60 | 0.0386 | 0.39 | 5.6 | 460 | 18.1 |
| 223 | -50.0 | -58 | 0.0407 | 0.41 | 5.9 | 445 | 17.5 |
| 224 | -49.4 | -57 | 0.0421 | 0.42 | 6.1 | 435 | 17.1 |
| 224 | -48.9 | -56 | 0.0434 | 0.43 | 6.3 | 424 | 16.7 |
| 225 | -48.3 | -55 | 0.0448 | 0.45 | 6.5 | 414 | 16.3 |
| 225 | -47.8 | -54 | 0.0469 | 0.47 | 6.8 | 398 | 15.7 |
| 226 | -47.2 | -53 | 0.0483 | 0.48 | 7.0 | 388 | 15.3 |
| 226 | -46.7 | -52 | 0.0496 | 0.50 | 7.2 | 378 | 14.9 |
| 227 | -46.1 | -51 | 0.0510 | 0.51 | 7.4 | 367 | 14.5 |
| 228 | -45.6 | -50 | 0.0531 | 0.53 | 7.7 | 352 | 13.9 |
| 228 | -45.0 | -49 | 0.0545 | 0.54 | 7.9 | 342 | 13.4 |
| 229 | -44.4 | -48 | 0.0565 | 0.57 | 8.2 | 326 | 12.8 |
| 229 | -43.9 | -47 | 0.0579 | 0.58 | 8.4 | 316 | 12.4 |
| 230 | -43.3 | -46 | 0.0600 | 0.60 | 8.7 | 300 | 11.8 |
| 230 | -42.8 | -45 | 0.0621 | 0.62 | 9.0 | 285 | 11.2 |
| 231 | -42.2 | -44 | 0.0634 | 0.63 | 9.2 | 274 | 10.8 |
| 231 | -41.7 | -43 | 0.0655 | 0.66 | 9.5 | 259 | 10.2 |
| 232 | -41.1 | -42 | 0.0676 | 0.68 | 9.8 | 243 | 9.6 |
| 233 | -40.6 | -41 | 0.0696 | 0.70 | 10.1 | 228 | 9.0 |
| 233 | -40.0 | -40 | 0.0717 | 0.72 | 10.4 | 212 | 8.4 |
| 234 | -39.4 | -39 | 0.0738 | 0.74 | 10.7 | 197 | 7.7 |
| 234 | -38.9 | -38 | 0.0758 | 0.76 | 11.0 | 181 | 7.1 |
| 235 | -38.3 | -37 | 0.0786 | 0.79 | 11.4 | 161 | 6.3 |
| 235 | -37.8 | -36 | 0.0807 | 0.81 | 11.7 | 145 | 5.7 |
| 236 | -37.2 | -35 | 0.0834 | 0.83 | 12.1 | 124 | 4.9 |
| 236 | -36.7 | -34 | 0.0855 | 0.85 | 12.4 | 109 | 4.3 |
| 237 | -36.1 | -33 | 0.0883 | 0.88 | 12.8 | 88 | 3.5 |
| 238 | -35.6 | -32 | 0.0903 | 0.90 | 13.1 | 73 | 2.9 |
| 238 | -35.0 | -31 | 0.0931 | 0.93 | 13.5 | 52 | 2.0 |
| 239 | -34.4 | -30 | 0.0958 | 0.96 | 13.9 | 31 | 1.2 |
| 239 | -33.9 | -29 | 0.0986 | 0.99 | 14.3 | 11 | 0.4 |
| Gauge pressure2) | |||||||
| (psig) | |||||||
| 240 | -33.3 | -28 | 0.1014 | 1.01 | 14.7 | 0 | |
| 240 | -32.8 | -27 | 0.1041 | 1.04 | 15.1 | 0.4 | |
| 241 | -32.2 | -26 | 0.1076 | 1.08 | 15.6 | 0.9 | |
| 241 | -31.7 | -25 | 0.1103 | 1.10 | 16.0 | 1.3 | |
| 242 | -31.1 | -24 | 0.1131 | 1.13 | 16.4 | 1.7 | |
| 243 | -30.6 | -23 | 0.1165 | 1.17 | 16.9 | 2.2 | |
| 243 | -30.0 | -22 | 0.1193 | 1.19 | 17.3 | 2.6 | |
| 244 | -29.4 | -21 | 0.1227 | 1.23 | 17.8 | 3.1 | |
| 244 | -28.9 | -20 | 0.1262 | 1.26 | 18.3 | 3.6 | |
| 245 | -28.3 | -19 | 0.1296 | 1.30 | 18.8 | 4.1 | |
| 245 | -27.8 | -18 | 0.1331 | 1.33 | 19.3 | 4.6 | |
| 246 | -27.2 | -17 | 0.1365 | 1.37 | 19.8 | 5.1 | |
| 246 | -26.7 | -16 | 0.1400 | 1.40 | 20.3 | 5.6 | |
| 247 | -26.1 | -15 | 0.1441 | 1.44 | 20.9 | 6.2 | |
| 248 | -25.6 | -14 | 0.1475 | 1.48 | 21.4 | 6.7 | |
| 248 | -25.0 | -13 | 0.1517 | 1.52 | 22.0 | 7.3 | |
| 249 | -24.4 | -12 | 0.1558 | 1.56 | 22.6 | 7.9 | |
| 249 | -23.9 | -11 | 0.1600 | 1.60 | 23.2 | 8.5 | |
| 250 | -23.3 | -10 | 0.1634 | 1.63 | 23.7 | 9 | |
| 250 | -22.8 | -9 | 0.1682 | 1.68 | 24.4 | 9.7 | |
| 251 | -22.2 | -8 | 0.1724 | 1.72 | 25.0 | 10.3 | |
| 251 | -21.7 | -7 | 0.1765 | 1.77 | 25.6 | 10.9 | |
| 252 | -21.1 | -6 | 0.1813 | 1.81 | 26.3 | 11.6 | |
| 253 | -20.6 | -5 | 0.1855 | 1.85 | 26.9 | 12.2 | |
| 253 | -20.0 | -4 | 0.1903 | 1.90 | 27.6 | 12.9 | |
| 254 | -19.4 | -3 | 0.1951 | 1.95 | 28.3 | 13.6 | |
| 254 | -18.9 | -2 | 0.1999 | 2.00 | 29.0 | 14.3 | |
| 255 | -18.3 | -1 | 0.2048 | 2.05 | 29.7 | 15 | |
| 255 | -17.8 | 0 | 0.2096 | 2.10 | 30.4 | 15.7 | |
| 256 | -17.2 | 1 | 0.2151 | 2.15 | 31.2 | 16.5 | |
| 256 | -16.7 | 2 | 0.2199 | 2.20 | 31.9 | 17.2 | |
| 257 | -16.1 | 3 | 0.2255 | 2.25 | 32.7 | 18 | |
| 258 | -15.6 | 4 | 0.2310 | 2.31 | 33.5 | 18.8 | |
| 258 | -15.0 | 5 | 0.2365 | 2.36 | 34.3 | 19.6 | |
| 259 | -14.4 | 6 | 0.2420 | 2.42 | 35.1 | 20.4 | |
| 259 | -13.9 | 7 | 0.2475 | 2.48 | 35.9 | 21.2 | |
| 260 | -13.3 | 8 | 0.2537 | 2.54 | 36.8 | 22.1 | |
| 260 | -12.8 | 9 | 0.2592 | 2.59 | 37.6 | 22.9 | |
| 261 | -12.2 | 10 | 0.2654 | 2.65 | 38.5 | 23.8 | |
| 261 | -11.7 | 11 | 0.2717 | 2.72 | 39.4 | 24.7 | |
| 262 | -11.1 | 12 | 0.2779 | 2.78 | 40.3 | 25.6 | |
| 263 | -10.6 | 13 | 0.2841 | 2.84 | 41.2 | 26.5 | |
| 263 | -10.0 | 14 | 0.2910 | 2.91 | 42.2 | 27.5 | |
| 264 | -9.4 | 15 | 0.2972 | 2.97 | 43.1 | 28.4 | |
| 264 | -8.9 | 16 | 0.3041 | 3.04 | 44.1 | 29.4 | |
| 265 | -8.3 | 17 | 0.3110 | 3.11 | 45.1 | 30.4 | |
| 265 | -7.8 | 18 | 0.3178 | 3.18 | 46.1 | 31.4 | |
| 266 | -7.2 | 19 | 0.3254 | 3.25 | 47.2 | 32.5 | |
| 266 | -6.7 | 20 | 0.3323 | 3.32 | 48.2 | 33.5 | |
| 267 | -6.1 | 21 | 0.3399 | 3.40 | 49.3 | 34.6 | |
| 268 | -5.6 | 22 | 0.3475 | 3.47 | 50.4 | 35.7 | |
| 268 | -5.0 | 23 | 0.3551 | 3.55 | 51.5 | 36.8 | |
| 269 | -4.4 | 24 | 0.3627 | 3.63 | 52.6 | 37.9 | |
| 269 | -3.9 | 25 | 0.3702 | 3.70 | 53.7 | 39 | |
| 270 | -3.3 | 26 | 0.3785 | 3.79 | 54.9 | 40.2 | |
| 270 | -2.8 | 27 | 0.3868 | 3.87 | 56.1 | 41.4 | |
| 271 | -2.2 | 28 | 0.3951 | 3.95 | 57.3 | 42.6 | |
| 271 | -1.7 | 29 | 0.4033 | 4.03 | 58.5 | 43.8 | |
| 272 | -1.1 | 30 | 0.4116 | 4.12 | 59.7 | 45 | |
| 278 | 4.4 | 40 | 0.5054 | 5.05 | 73.3 | 58.6 | |
| 283 | 10.0 | 50 | 0.6150 | 6.15 | 89.2 | 74.5 | |
| 289 | 15.6 | 60 | 0.7419 | 7.42 | 107.6 | 92.9 | |
| 294 | 21.1 | 70 | 0.8880 | 8.88 | 128.8 | 114.1 | |
| 298 | 24.4 | 76 | 0.9860 | 9.86 | 143.0 | 128.3 | |
| 298 | 25.0 | 77 | 1.00 | 10.0 | 145.4 | 130.7 | |
| 299 | 25.6 | 78 | 1.02 | 10.2 | 147.9 | 133.2 | |
| 299 | 26.1 | 79 | 1.04 | 10.4 | 150.5 | 135.8 | |
| 300 | 26.7 | 80 | 1.05 | 10.5 | 153.0 | 138.3 | |
| 300 | 27.2 | 81 | 1.07 | 10.7 | 155.6 | 140.9 | |
| 301 | 27.8 | 82 | 1.09 | 10.9 | 158.3 | 143.6 | |
| 301 | 28.3 | 83 | 1.11 | 11.1 | 161.0 | 146.3 | |
| 302 | 28.9 | 84 | 1.13 | 11.3 | 163.7 | 149.0 | |
| 303 | 29.4 | 85 | 1.15 | 11.5 | 166.4 | 151.7 | |
| 303 | 30.0 | 86 | 1.17 | 11.7 | 169.2 | 154.5 | |
| 304 | 30.6 | 87 | 1.19 | 11.9 | 172.0 | 157.3 | |
| 304 | 31.1 | 88 | 1.21 | 12.1 | 174.8 | 160.1 | |
| 305 | 31.7 | 89 | 1.23 | 12.3 | 177.7 | 163.0 | |
| 305 | 32.2 | 90 | 1.25 | 12.5 | 180.6 | 165.9 | |
| 306 | 32.8 | 91 | 1.27 | 12.7 | 183.6 | 168.9 | |
| 306 | 33.3 | 92 | 1.29 | 12.9 | 186.6 | 171.9 | |
| 307 | 33.9 | 93 | 1.31 | 13.1 | 189.6 | 174.9 | |
| 308 | 34.4 | 94 | 1.33 | 13.3 | 192.7 | 178.0 | |
| 308 | 35.0 | 95 | 1.35 | 13.5 | 195.8 | 181.1 | |
| 309 | 35.6 | 96 | 1.37 | 13.7 | 198.9 | 184.2 | |
| 309 | 36.1 | 97 | 1.39 | 13.9 | 202.1 | 187.4 | |
| 310 | 36.7 | 98 | 1.42 | 14.2 | 205.3 | 190.6 | |
| 310 | 37.2 | 99 | 1.44 | 14.4 | 208.6 | 193.9 | |
| 311 | 37.8 | 100 | 1.46 | 14.6 | 211.9 | 197.2 | |
| 311 | 38.3 | 101 | 1.48 | 14.8 | 215.2 | 200.5 | |
| 312 | 38.9 | 102 | 1.51 | 15.1 | 218.6 | 203.9 | |
| 313 | 39.4 | 103 | 1.53 | 15.3 | 222.0 | 207.3 | |
| 313 | 40.0 | 104 | 1.55 | 15.5 | 225.4 | 210.7 | |
| 314 | 40.6 | 105 | 1.58 | 15.8 | 228.9 | 214.2 | |
| 314 | 41.1 | 106 | 1.60 | 16.0 | 232.5 | 217.8 | |
| 315 | 41.7 | 107 | 1.63 | 16.3 | 236.0 | 221.3 | |
| 315 | 42.2 | 108 | 1.65 | 16.5 | 239.7 | 225.0 | |
| 316 | 42.8 | 109 | 1.68 | 16.8 | 243.3 | 228.6 | |
| 316 | 43.3 | 110 | 1.70 | 17.0 | 247.0 | 232.3 | |
| 317 | 43.9 | 111 | 1.73 | 17.3 | 250.8 | 236.1 | |
| 318 | 44.4 | 112 | 1.75 | 17.5 | 254.5 | 239.8 | |
| 318 | 45.0 | 113 | 1.78 | 17.8 | 258.4 | 243.7 | |
| 319 | 45.6 | 114 | 1.81 | 18.1 | 262.2 | 247.5 | |
| 319 | 46.1 | 115 | 1.84 | 18.4 | 266.2 | 251.5 | |
| 320 | 46.7 | 116 | 1.86 | 18.6 | 270.1 | 255.4 | |
| 320 | 47.2 | 117 | 1.89 | 18.9 | 274.1 | 259.4 | |
| 321 | 47.8 | 118 | 1.92 | 19.2 | 278.2 | 263.5 | |
| 321 | 48.3 | 119 | 1.95 | 19.5 | 282.3 | 267.6 | |
| 322 | 48.9 | 120 | 1.97 | 19.7 | 286.4 | 271.7 | |
| 330 | 56.9 | 134 | 2.42 | 24.2 | 351.1 | 336.4 | |
| 350 | 76.9 | 170 | 3.87 | 38.7 | 560.7 | 546.0 | |
| 370 | 96.9 | 206 | 5.88 | 58.8 | 852.5 | 837.8 | |
| 390 | 117 | 242 | 8.60 | 86.0 | 1248 | 1233 | |
| 400 | 127 | 260 | 10.3 | 103 | 1495 | 1480 | |
| 405 | 132 | 270 | 11.3 | 113 | 1645 | 1630 | |
1) Between -60 oF (-51 oC) to -28 oF (-33 oC) the vacuum pressure values in the seventh and eighth column are represented in mm and inches Mercury
2) The gauge numbers shown in that seventh column all are vs atmospheric pressure at sea level (14.7 psia)



