Air Ducts - Major Friction Loss vs. Temperature and Pressure
The influence of temperature and air pressure on major friction loss.
Air ducts friction loss and pressure drop diagrams are normally made for air at Normal Temperature and Pressure (NTP) condition - with a temperature of 20 oC and a atmospheric pressure of 1 bar.
Influence of Temperature
The friction coefficient in the D'Arcy-Weisbach Equation increases with higher temperature but the coefficient will also be outweighed by the reduced pressure loss due to reduced density of the air.
- the pressure loss in an air duct is reduced with higher temperature
With air temperature other than 20 oC - a "Temperature Compensating Coefficient" can be used to modify the pressure loss:
Δp = kt ΔpNTP (1)
where
Δp = actual friction loss (pressure or head)
kt = Temperature Compensating Coefficient
ΔpNTP = friction loss at NTP conditions (pressure or head)
"Temperature Compensating Coefficient" for various air temperatures:
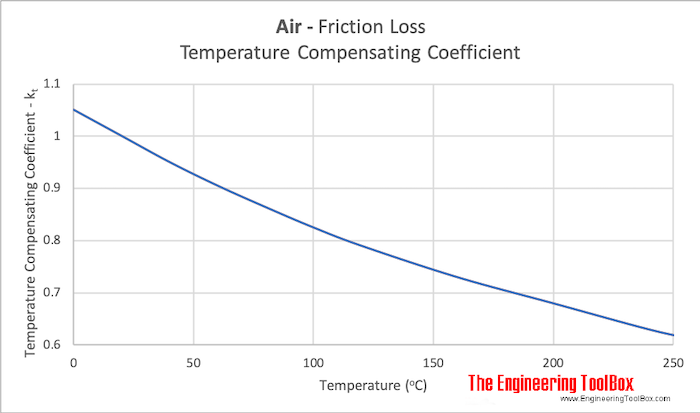
Example - Air with Temperature 100 oC
For air with temperature 100 oC the "Temperature Compensating Coefficient" is approximately 0.83. So,
- the pressure loss in air with temperature 100 oC is approximately 83 % of the pressure loss in air at standard NTP conditions 20 oC
Influence of Pressure
If the actual pressure differ from atmospheric pressure (1 bar abs) - friction loss in air ducts should be compensated with a "Pressure Compensating Coefficient":
Δp = kp ΔpNTP (2)
where
Δp = actual friction loss (pressure or head)
kp = Pressure Compensating Coefficient
ΔpNTP = friction loss at NTP conditions (pressure or head)
The "Pressure Compensating Coefficient" can be estimated for various pressures from the diagram below:
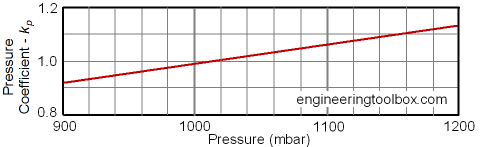
- 1000 mbar = 1 bar = 105 Pa (N/m2) = 0.1 N/mm2 = 10,197 kp/m2 = 10.20 m H2O = 0.9869 atm = 14.50 psi (lbf/in2) = 106 dyn/cm2 = 750 mmHg



