Air - Molecular Weight and Composition
Dry air is a mixture of gases where the average molecular weight (or molar mass) can be calculated by adding the weight of each component.
The molecular weight (or molar mass) of a substance is the mass of one mole of the substance, and can be calculated by summarizing the molar masses of all the atoms in the molecule.
Components in Dry Air
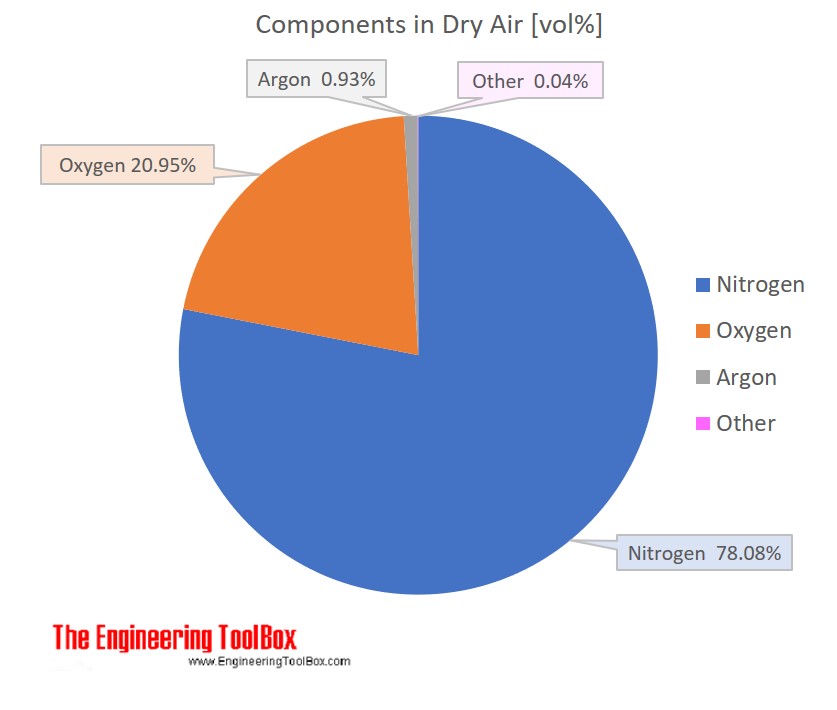
Air is a mixture of several gases, where the two most dominant components in dry air are 21 vol% oxygen and 78 vol% nitrogen . Oxygen has a molar mass of 15.9994 g/mol and nitrogen has a molar mass of 14.0067 g/mol. Since both of these elements are diatomic in air - O2 and N2, the molar mass of oxygen gas is aprox. 32 g/mol and the molar mass of nitrogen gas is aprox. 28 g/mol.
The average molar mass is equal to the sum of the mole fractions of each gas multiplied by the molar mass of that particular gas:
Mmixture = x1 M1 + ......+ xn Mn (1)
where
xi = mole fractions of each gas
Mi = the molar mass of each gas
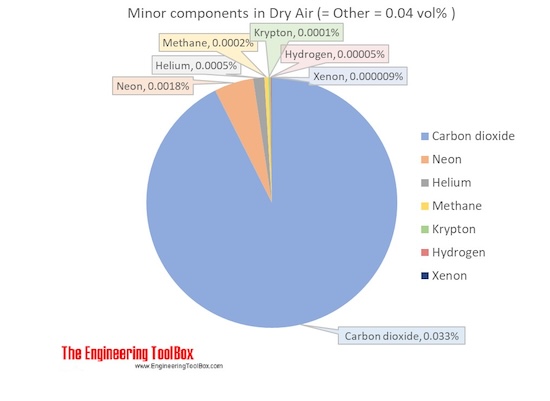
The molar mass of dry air with oxygen, nitrogen and the other components as indicated below is 28.9647 g/mol. Composition and content of each gas in air is given in the figures and the table below.
See also Air Density at varying pressure, Density and specific weight at varying temperature, Diffusion Coefficients for Gases in Air, Dynamic (absolute) and kinematic viscosity, Prandtl Number, Specific heat at varying temperature and Specific heat at varying pressure, Thermal Conductivity, Thermal Diffusivity, Properties at gas-liquid equilibrium conditions and Air properties, for other properties of air.
For full table with Molar mass an Molar mass in Air - rotate the screen!
| Components in dry air | Volume ratio = Molar ratio, compared to dry air | Molar Mass | Molar mass in air | |||
|---|---|---|---|---|---|---|
| Name | Formula | (mol/molair) | (vol %) | (g/mol) (kg/kmol) | (g/molair) (kg/kmolair) | (wt %) |
| Nitrogen | N2 | 0.78084 | 78.084 | 28.013 | 21.873983 | 75.52 |
| Oxygen | O2 | 0.20946 | 20.946 | 31.999 | 6.702469 | 23.14 |
| Argon | Ar | 0.00934 | 0.934 | 39.948 | 0.373114 | 1.29 |
| Carbon dioxide | CO2 | 0.00033 | 0.033 | 44.010 | 0.014677 | 0.051 |
| Neon | Ne | 0.00001818 | 0.001818 | 20.180 | 0.000367 | 0.0013 |
| Helium | He | 0.00000524 | 0.000524 | 4.003 | 0.000021 | 0.00007 |
| Methane | CH4 | 0.00000179 | 0.000179 | 16.042 | 0.000029 | 0.00010 |
| Krypton | Kr | 0.0000010 | 0.0001 | 83.798 | 0.000084 | 0.00029 |
| Hydrogen | H2 | 0.0000005 | 0.00005 | 2.016 | 0.000001 | 0.000003 |
| Xenon | Xe | 0.00000009 | 0.000009 | 131.293 | 0.000012 | 0.00004 |
| Average molar mass of air | 28.9647 | |||||
- 1 kg = 2.2046 lb
Air Density
The density of dry air can be calculated with the Ideal Gas Law
ρ = P / (R T) (1)
where
P = pressure (Pa)
Rair = 287.05 = individual gas constant (J/kg K)
T = absolute temperature (K)
Example: The density of dry air at atmospheric pressure 101.325 kPa (101325 Pa) and 0 oC (273.15 K) can be calculated as
ρ = (101325 Pa) / ((287.05 J/kg K) (273.15 K))
= 1.292 (kg/m3)
Water Vapor
Water vapor is almost always present in air. The content may vary and the maximum amount possible of water vapor in dry air depends on the temperature of the air.
Water vapor - H2O - is composed of one oxygen atom and two hydrogen atoms. Hydrogen is the lightest element with a molar mass of 1 g/mol, while oxygen has 16 g/mol . Thus, the water vapor atom has an molar mass of 18 g/mol, and water vapor is lighter than O2with 32 g/mol and N2with 28 g/mol .
- Note! Water vapor in air will dilute the other gases and reduce the total density of the mixture. Dry air is more dense than humid air!
The vapor in air may saturate to droplets when temperature is decreased or pressure is increased.
Humid air containing water molecules as liquid - droplets - may be more dense than dry air or humid air containing water only as vapor.



