Ethanol Freeze Protected Water Solutions
Freezing temperature and flash points for ethanol based water solutions or brines.
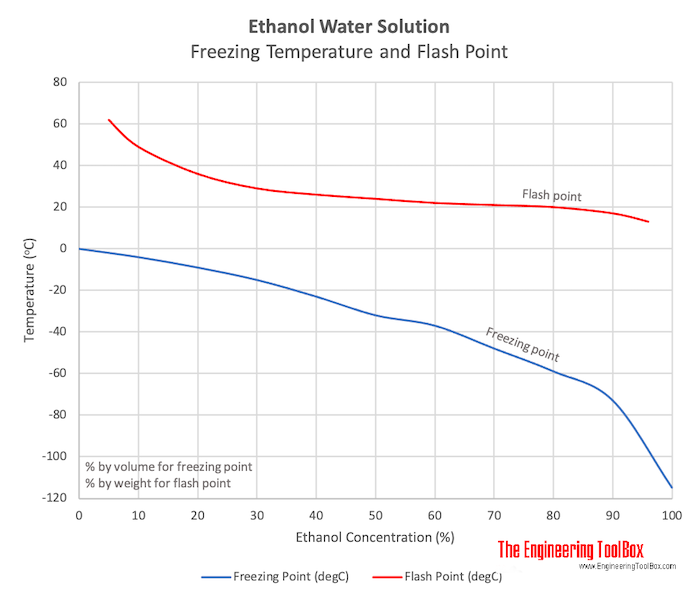
Ethanol based Water Solutions Freezing Point
| Ethanol Concentration (% by volume) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freezing Point | (oF) | 32 | 25 | 15 | 5 | -10 | -25 | -35 | -55 | -75 | -110 | -175 |
| (oC) | 0 | -4 | -9 | -15 | -23 | -32 | -37 | -48 | -59 | -73 | -115 | |
Flash Points of Ethanol based Water Solutions
The flash point of a chemical is the lowest temperature where it will evaporate enough fluid to form a combustible concentration of gas. The flash point is an indication of how easy a chemical may burn.
| Ethanol Concentration (% by weight) | 5 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 96 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flash Point | (oF) | 144 | 120 | 97 | 84 | 79 | 75 | 72 | 70 | 68 | 63 | 55 |
| (oC) | 62 | 49 | 36 | 29 | 26 | 24 | 22 | 21 | 20 | 17 | 13 | |
Warning
- HIGH flammability if pure
Download and print Ethanol Water Solution - Freezing Temperature and Flash Point chart.
Alternatives to Ethanol based Water Solutions
- Ethylene glycol based water solutions
- Propylene glycol based water solutions
- Methanol based water solutions
Example - Ethanol Concentration at Freezing Point at -20 oC
By using linear interpolation between two known concentrations and their freezing points - the concentration at a third freezing point can be calculated as
CC = ((CB - CA) / (tB - tA)) (tC - tA) + CA (1)
where
C = concentration in ethanol - water solution
t = freezing point (oC, oF)
A, B = known freezing points
C = calculated freezing point
The ethanol concentration with freezing point at -20 oC can be calculated by interpolating the concentration between freezing point -15 oC and -23 oC in the table above.
Cc = (((40 %) - (30 %)) / ((-23 oC) - (-15 oC))) ((-20 oC) - (-15 oC)) + (30 %) = 36.25 %
Note that this calculation is simplified by assuming that the concentration vs. freezing point follows a straight line. This not necessary correct.
If a 90% ethanol-water solution shall be mixed with clean water to achieve a freezing point of -20 oC (ethanol concentration 36.25% (0.3625)) - the amount of added water can be calculated with volume balance - the amount of ethanol before mix is the same as after the mix:
C Vs = Cm (Vs + Vw) (2)
where
Vs = volume of the ethanol - water solution (liter, gallon)
Cm = concentration in mix
Vm = volume of mix (liter, gallon)
Vw = volume of the added clean water (liter, gallon)
Rearranging the equation to express the volume of water added to the mixture
Vw = (C - Cm) Vs / Cm (2b)
Substituting with values
Vw = (0.9 - 0.3625) Vs / 0.3625
= 1.48 Vs
- for every liter 90% ethanol-water solution 1.48 liter of clean water must be mixed in to achieve an ethanol concentration of 36.25 % and freezing point -20 oC.