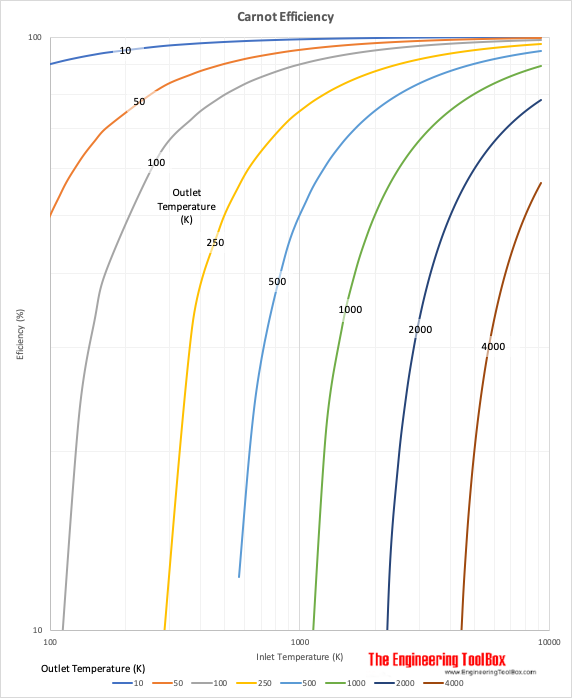
Carnot Cycle Efficiency
The efficiency of the Carnot cycle.
An ideal reversible cycle where heat is taken in at constant upper temperature and rejected at constant lower temperature was suggested by Sadi Carnot. The theoretically most efficient heat engine cycle, the Carnot cycle, consists of
- two isothermal processes and
- two adiabatic processes

Since the second law of thermodynamics states that not all supplied heat in a heat engine can be used to do work the Carnot efficiency limits the fraction of heat that can be used.
The Carnot efficiency can be expressed as
μC = (Ti - To) / Ti (1)
where
μC = efficiency of the Carnot cycle
Ti = temperature at the engine inlet (K)
To = temperature at engine exhaust (K)
The wider the range of temperature, the more efficient becomes the cycle. The lowest temperature is limited by the temperature of the sink of heat - if it is the atmosphere or the ocean, river or whatever available. Normally the lowest temperature available is in the range 10 - 20 oC. The maximum temperature is limited by the metallurgical strength of the available materials.