Hydrocarbons - Autoignition Temperatures and Flash Points
Autoignition temperatures and flash points (°C and °F) of different types of hydrocarbons with varying carbon numbers up to C12.
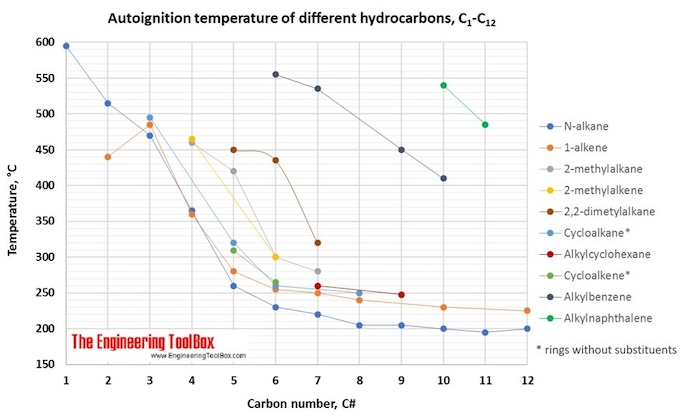
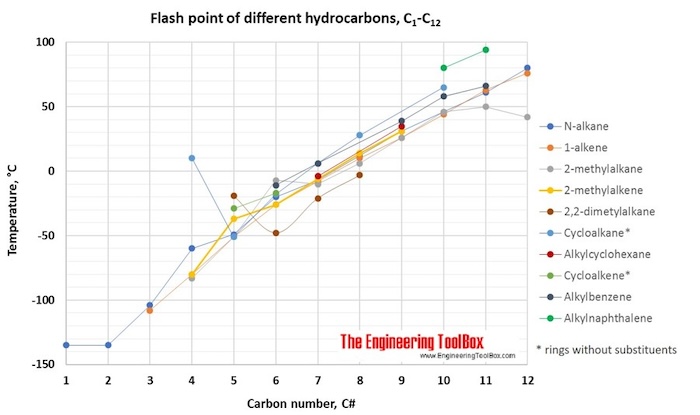
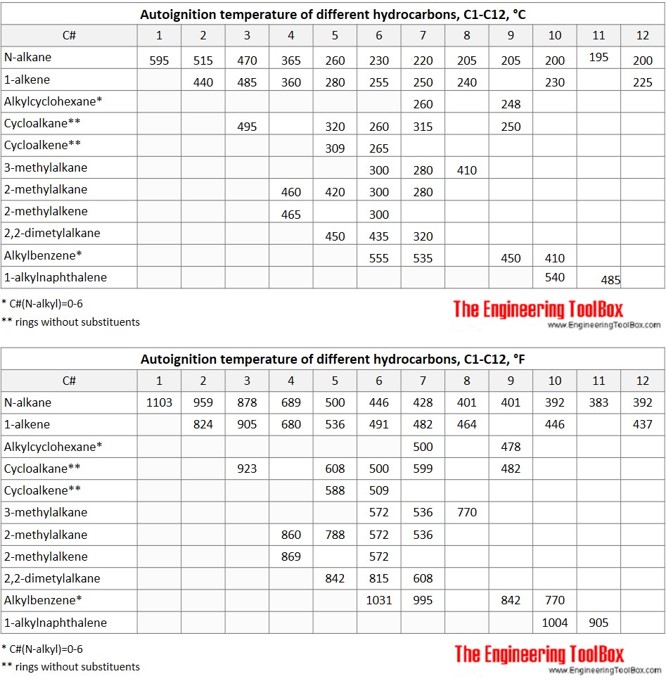
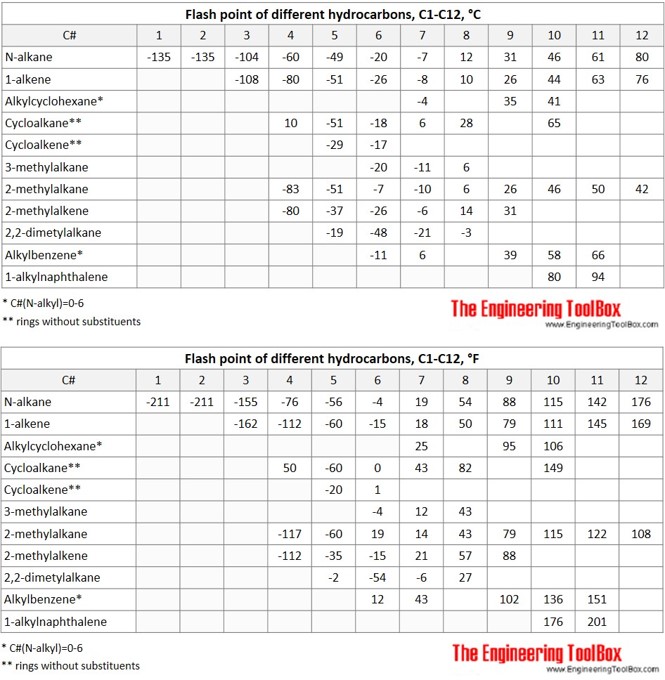
The tables and figures below show the autoignition temperature (AIT) and flash point (FP) for different hydrocarbons. In the figures, the AIT and FP are shown as function of number of carbon atoms in the hydrocarbons, which are grouped as n-alkanes, 1-alkenes, 2-methylalkanes, 3-methylalkanes, 2,2,dimethylalkanes, 2-methylalkenes, cycloalkanes, alkylcyclohexanes, cycloalkenes, alkylbenzenes and alkylnaphthalenes.
- Autoignition temperature = kindling point
- is the temperature at which a material spontaneously ignites in a normal atmosphere without an external source of ignition.
- is the temperature required to supply the activation energy for combustion
- is usually applied to combustible fuel mixtures
- Flash point - the lowest temperature at which vapour of a volatile material can be ignited whit an ignition source present
- Values of autoignition temperature are generally higher than flash point, as given for pure hydrocarbons in the tables and figures below.
- With an increase in pressure the autoignition temperature decreases. This is particularly important from a safety point of view when hydrocarbons are compressed.
- Hydrocarbons with high vapor pressures (lighter compounds) have low flash points. Generally, flash point increases with an increase in boiling point.
- Flash point is an important parameter for safety considerations, especially during storage and transportation of volatile petroleum products (i.e., LPG, light naphtha, gasoline) in a high-temperature environment. The surrounding temperature around a storage tank should always be less than the flash point of the fuel to avoid possibility of ignition.
- Flash point should not be mistaken with fire point, which is defined as the minimum temperature at which the hydrocarbon will continue to burn for at least 5 s after being ignited by a flame.
- Temperature classification of equipment in hazardous areas are related to surrounding substances auto-ignition temperature.
See also Flash Point - Fuels
| IUPAC name | Common name | Flash point | Autoignition temp | ||
| °C | °F | °C | °F | ||
| Benzene | Benzol | -11 | 12 | 555 | 1031 |
| But-1-ene | 1-butene, 1-butylene, Ethylethylene | -80 | -112 | 360 | 680 |
| But-2-ene | Cis-2-butene | -72 | -98 | 325 | 617 |
| But-2-ene | Trans-2-butene | -73 | -99 | 324 | 615 |
| 1,2-butadiene | <-50 | <-58 | 340 | 644 | |
| 1,3-butadiene | Biethylene, divinylbutadiene, pyrrolylene, vinylethylene | -85 | -121 | 415 | 779 |
| N-butane | -60 | -76 | 365 | 689 | |
| Butylbenzene | 58 | 136 | 410 | 770 | |
| Butylcyclohexane | 41 | 106 | |||
| Butylcyclopentane | 32 | 90 | |||
| 1-butyne | Ethylacetylene | <-14 | <6.8 | ||
| 2-butyne | Crotonylene | -25 | -13 | ||
| Cyclobutane | <10 | <50 | |||
| Cyclodecane | 65 | 149 | |||
| Cycloheptane | 6 | 43 | |||
| 1,3-cyclohexadiene | -8 | 18 | |||
| Cyclohexane | Hexamethylene, hexanaphthene | -18 | 0 | 260 | 500 |
| Cyclohexene | -17 | 1 | 265 | 509 | |
| Cyclohexylbenzene | 99 | 210 | |||
| Cyclooctane | 28 | 82 | 250 | 482 | |
| Cyclopentane | -51 | -60 | 320 | 608 | |
| Cyclopentene | -29 | -20 | 309 | 588 | |
| Cyclopropane | Trimethylene | 495 | 923 | ||
| o-cymene | 53 | 127 | |||
| N-decane | 46 | 115 | 200 | 392 | |
| 2-decanol | 85 | 185 | |||
| 1-decene | 44 | 111 | 230 | 446 | |
| 2,3-dimethyl-2-butene | Tetramethylethylene | <-20 | <-4 | 400 | 752 |
| 1,2-dimethylbenzen | orto-xylene | 30 | 86 | 465 | 869 |
| 1,3-dimethylbenzen | meta-xylene | 25 | 77 | 540 | 1004 |
| 1,4-dimethylbenzen | para-xylene | 25 | 77 | 540 | 1004 |
| 2,2-dimethylbutane | Neohexane | -48 | -54 | 435 | 815 |
| 2,3-dimethylbutane | Diisopropyl | -29 | -20 | 415 | 779 |
| 2,2-dimethylhexane | -3 | 27 | |||
| 2,2-dimethylpentane | -21 | -6 | 320 | 608 | |
| 3,3-dimethylpentane | -15 | 5 | 320 | 608 | |
| 2,3-dimethylpentane | -12 | 10 | 330 | 626 | |
| 2,4-dimethylpentane | <-20 | <-4 | 325 | 617 | |
| 2,2-dimethylpropane | Neo-pentane, trimethylethane | -19 | -2 | 450 | 842 |
| Diphenyl methane | 130 | 266 | |||
| N-docosane | 211 | 412 | |||
| Docosanoic acid | Behenic acid | 176.3 | 349 | ||
| N-dodecane | 80 | 176 | 200 | 392 | |
| Dodecanoic acid | Lauric acid | 134.1 | 273 | ||
| 1-dodecanol | Lauryl alcohol | 119 | 246 | ||
| 1-dodecene | 76 | 169 | 225 | 437 | |
| N-eicosane | 187 | 369 | |||
| Ethane | -135 | -211 | 515 | 959 | |
| Ethene | Ethylene | 440 | 824 | ||
| Ethylbenzene | 15 |
59 |
|||
| Ethylcyclohexane | 22 |
72 |
|||
| Ethylcyclopentane | -4 |
25 |
|||
| Ethyne | Acetylene | 305 | 581 | ||
| N-heptacosane | 269 | 516 | |||
| N-heptadecane | 148 | 298 | |||
| N-heptane | -7 | 19 | 220 | 428 | |
| 2-heptanol | 59 | 138 | |||
| 1-heptene | 1-heptylene | -8 | 18 | 250 | 482 |
| Cis-2-heptene | -6 | 21 | |||
| Trans-2-heptene | -1 | 30 | |||
| Cis-3-heptene | -7 | 19 | 260 | 500 | |
| Trans-3-heptene | -6 | 21 | |||
| 1-heptyne | -2 | 28 | 245 | 473 | |
| N-hexadecane | 135 | 275 | |||
| N-hexane | Hexane | <-20 | <-4 | 230 | 446 |
| 3-hexanol | 41.7 | 107 | |||
| 1-hexene | -26 | -15 | 255 | 491 | |
| Cis-2-hexene | -25 | -13 | 244 | 471 | |
| Trans-2-hexene | -25 | -13 | 244 | 471 | |
| Hexylbenzene | 80 | 176 | |||
| 1-hexyne | -20 | -4 | 263 | 505 | |
| 3-hexyne | -14 | 7 | |||
| Isobutylbenzene | 55 | 131 | |||
| Isopropylbenzene | Cumene | 31 | 88 | 420 | 788 |
| Methane | -135 | -211 | 595 | 1103 | |
| 2-methyl-1,3-butadiene | Isoprene | -54 | -65 | 220 | 428 |
| 2-methyl-1-butene | -37 | -35 | |||
| 3-methyl-1-butene | -58 | -72 | 365 | 689 | |
| 1-Methyl-1-cyclohexene | -4 | 25 | |||
| 4-Methyl-1-cyclohexene | -1 | 30 | |||
| 2-methyl-1-heptene | 14 | 57 | |||
| 2-methyl-1-hexene | -6 | 21 | |||
| 2-methyl-1-octene | 31 | 88 | |||
| 2-methyl-1-pentene | 1-methyl-1-propylethylene | -26 | -15 | 300 | 572 |
| 2-methyl-1-propene | Isobutene, Isobutylene | -80 | -112 | 465 | 869 |
| 2-methyl-2-butene | Trimethylethylene, beta-iso-amylene | -45 | -49 | 290 | 554 |
| Methylbenzene | Toluene, toluol | 6 | 43 | 535 | 995 |
| 2-methylbutane | Iso-pentane | -51 | -60 | 420 | 788 |
| Methylcyclohexane | Heptanaphthene | -4 | 25 | 260 | 500 |
| Methylcyclopentane | <-10 | <14 | 315 | 599 | |
| 2-methyldecane | 50 | 122 | |||
| 2-methylheptane | 6 | 43 | |||
| 3-methylheptane | 6 | 43 | 410 | 770 | |
| 4-methylheptane | 6 | 43 | |||
| 2-methylhexane | -10 | 14 | 280 | 536 | |
| 3-methylhexane | -11 | 12 | 280 | 536 | |
| 1-methylnaphthalene | 94 | 201 | 485 | 905 | |
| 2-methylnaphthalene | 98 | 208 | 488 | 910 | |
| 2-methylnonane | iso-decane | 46 | 115 | ||
| 2-methyloctane | isononane, dimethylheptane | 26 | 79 | ||
| 2-methylpentane | iso-hexane, i-caproylhydride | <-7 | <19.4 | 300 | 572 |
| 3-methylpentane | Diethylmethylmethane | <-20 | <-4 | 300 | 572 |
| 2-methylpropane | iso-butane | -83 | -117 | 460 | 860 |
| 2-methylundecane | 42 | 108 | |||
| Naphthalene | 80 | 176 | 540 | 1004 | |
| N-nonadecane | 168 | 334 | |||
| N-nonane | 31 | 88 | 205 | 401 | |
| 2-nonanol | 82.2 | 180 | |||
| 3-nonanol | 79.5 | 175 | |||
| 4-nonanol | 79.5 | 175 | |||
| 1-nonene | 26 | 79 | |||
| N-octadecane | 165 | 329 | |||
| N-octane | 12 | 54 | 205 | 401 | |
| 1-octanol | Capryl alcohol | 81 | 178 | 259 | 497 |
| 1-octene | 1-caprylene | 10 | 50 | 240 | 464 |
| N-pentadecane | 114 |
237 |
|||
| N-pentane | -49 | -56 | 260 | 500 | |
| 1-pentene | n-amylene, propylethylene | -51 | -60 | 280 | 536 |
| Pentylbenzene | Phenylpentane, amylbenzene | 66 | 151 | ||
| 1-pentyne | Propylacetylene | <-20 | <-4 | ||
| Phenanthrene | 171 | 340 | >450 | >842 | |
| Propane | -104 | -155 | 470 | 878 | |
| Propene | Propylene | -108 | -162 | 485 | 905 |
| Propylbenzene | 39 | 102 | 450 | 842 | |
| Propylcyclohexane | 35 | 95 | 248 | 478 | |
| Propylcyclopentane | 16 | 61 | |||
| Propyne | 340 | 644 | |||
| Pyrene | Benzo(def)phenanthrene | 200 | 392 | ||
| Styrene | 32 |
90 |
|||
| N-tetradecane | 100 |
212 |
|||
| N-tridecane | 79 | 174 | |||
| Tridecanoic acid | Tridecylic acid | 139.6 | 283 | ||
| 1,2,3-trimethylbenzene | 51 | 124 | |||
| 1,3,5-trimethylbenzene | Mesitylene, trimethylbenzol | 44 | 111 | 550 | 1022 |
| 2,2,4-trimethylpentane | Iso-octane | -9 | 16 | 410 | 770 |
| N-undecane | 61 | 142 | 195 | 383 | |
| Undecanoic acid | 128 | 262 | |||
| 3-undecanol | 94 | 201 | |||
| 1-undecene | 63 | 145 | |||
| o-xylene | 17 | 63 | |||
| m-xylene | 25 | 77 | |||
| p-xylene | 25 | 77 | |||
<
In oil industry, the following terms for the different hydrocarbons are used:
Paraffins: Alkenes - lineare, saturated molecules, straight or branched chains
Olefins: Alkenes - lineare, unsaturated molecules, straight or branched chains (these are normally not present in unprocessed crude oil)
Naphthenes: Cycloalkanes - saturated ring structures, or cycloalkenes - partly saturated ring structures, with or without substituents connected to the ring
Aromatics: Unsaturated 6-ring structures (3 doble bounds in one ring), with or without substituents
Related Topics
-
Material Properties
Properties of gases, fluids and solids. Densities, specific heats, viscosities and more. -
Risk, Reliability and Safety
Risk, reliability and safety in process control systems.
Related Documents
-
Acetone - Thermophysical Properties
Chemical, physical and thermal properties of acetone, also called 2-propanone, dimethyl ketone and pyroacetic acid. Phase diagram included. -
Ammonia - NH3 - Thermodynamic Properties
Thermodynamic properties of saturated and superheated ammonia R-717 like specific volume, enthalpy and entropy. -
Benzene - Thermophysical properties
Chemical, physical and thermal properties of benzene, also called benzol. Phase diagram included. -
Butane - Thermal Conductivity vs. Temperature and Pressure
Online calculators, figures and tables showing thermal conductivity of liquid and gaseous butane, C4H10, at varying temperature and pressure, SI and Imperial units. -
Crude Oil - Density vs. Temperature
Variations in crude oil density are shown as function of temperatur, together with volume correction factors. -
Densities of Aqueous Solutions of Organic Acids
Changes in density of aqueous solutions with changes in concentration at 20°C. Density of acetic acid, citric acid, formic acid, D-lactic acid, oxalic acid and trichloroacetic acid in water is plotted as function of wt%, mol/kg water and mol/l solution. -
Density of Aqueous Solutions of Organic Substances as Sugars and Alcohols
Changes in density of aqueous solutions with changes in concentration at 20°C. Density of some sugars, alcohols and other organic substances in water is plotted as function of wt%, mol/kg water and mol/l solution. -
Dowtherm A
Physical properties of Dowtherm A. -
Ethylene - Thermophysical Properties
Chemical, physical and thermal properties of ethylene, also called ethene, acetene and olefiant gas. Phase diagram included. -
Flash Points - Liquids
The flash points for some common liquids and fuels. -
Fuels and Chemicals - Autoignition Temperatures
Autoignition points for fuels and chemicals like butane, coke, hydrogen, petroleum and more. -
Gases - Explosion and Flammability Concentration Limits
Flame and explosion limits for gases like propane, methane, butane, acetylene and more. -
Hazard vs. Flash Points
The flash point of a chemical indicates how easy it may ignite and burn. -
Hazardous Areas - European Classification Standard
European hazardous area classification with zones, protection types, temperature codes and codes. -
Hazardous Areas - North America Classification
North American hazardous locations classification with classes, divisions and groups -
Hydrocarbons - Physical Data
Molweight, melting and boiling point, density, flash point and autoignition temperature, as well as number of carbon and hydrogen atoms in each molecule for 200 different hydrocarbons. -
Jet Fuel - Density vs. Temperature
Variations in jet fuel density as function of temperatur, together with volume correction factors. -
Methane - Dynamic and Kinematic Viscosity vs. Temperature and Pressure
Online calculator, figures and tables showing dynamic and kinematic viscosity of methane, CH4, at varying temperature and pressure - Imperial and SI Units. -
Methanol - Dynamic and Kinematic Viscosity vs. Temperature and Pressure
Online calculator, figures and tables showing dynamic and kinematic viscosity of liquid methanol,CH3OH, at varying temperature - Imperial and SI Units. -
Naming of Organic Compounds
Nomenclature rules for different groups of organic compounds and functional groups, together with examples of use of the rules. -
Yield Structure of Crude Oils with Increasing Density of Crude
Yields of different crude oil distillation cuts are plotted as function of whole crude specific gravity. Fractions based on European and North American markets, and the typical differences in crude oil fractionation in the two markets are also shown.




