Densities of Aqueous Solutions of Inorganic Chlorides
Changes in density of aqueous solutions with changes in concentration at 20°C. Density of inorganic chlorides in water is plotted as function of wt%, mol/kg water and mol/l solution.
Be aware of the concentration units in the figures:
wt%: Mass of solute/total mass of solution*100%
mol/kg: Molality = moles of solute/kg of water
mol/liter: Molarity = moles of solute/liter of solution
Values are tabulated below the figures.
See also density of aqueous solutions of inorganic potassium salts, inorganic sodium salts, some other inorganic substances, organic acids and organic substances as sugars and alcohols.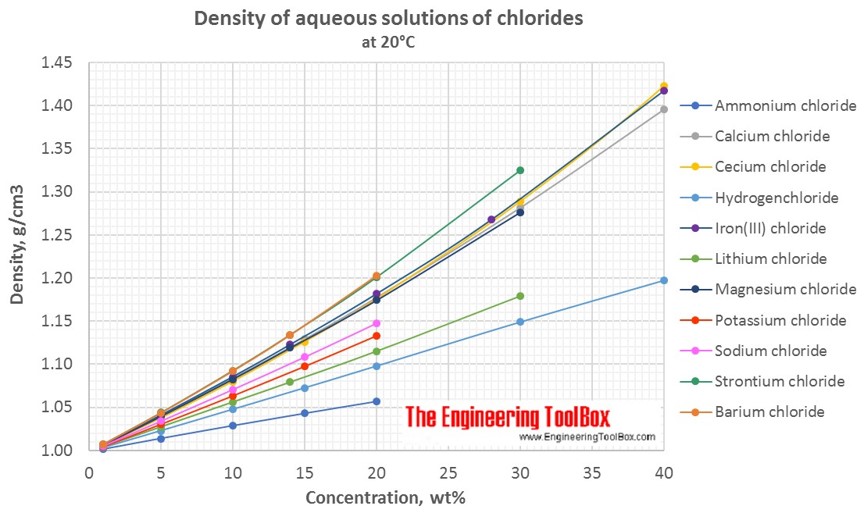
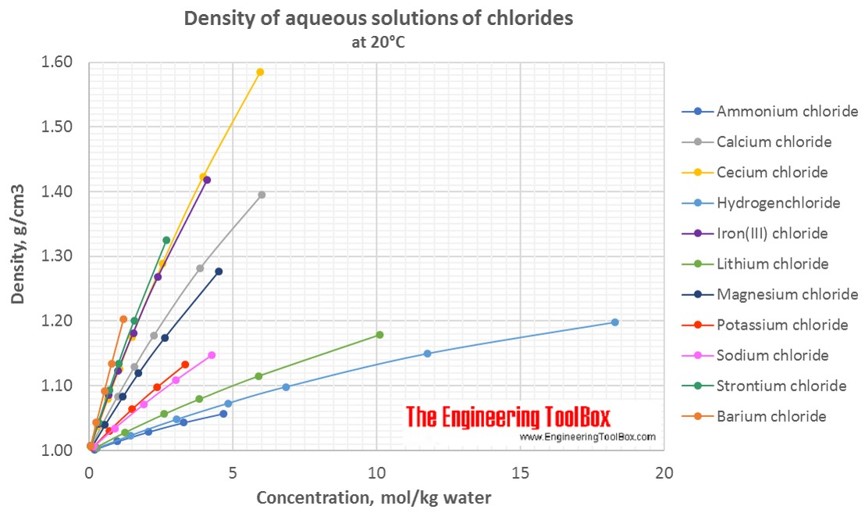

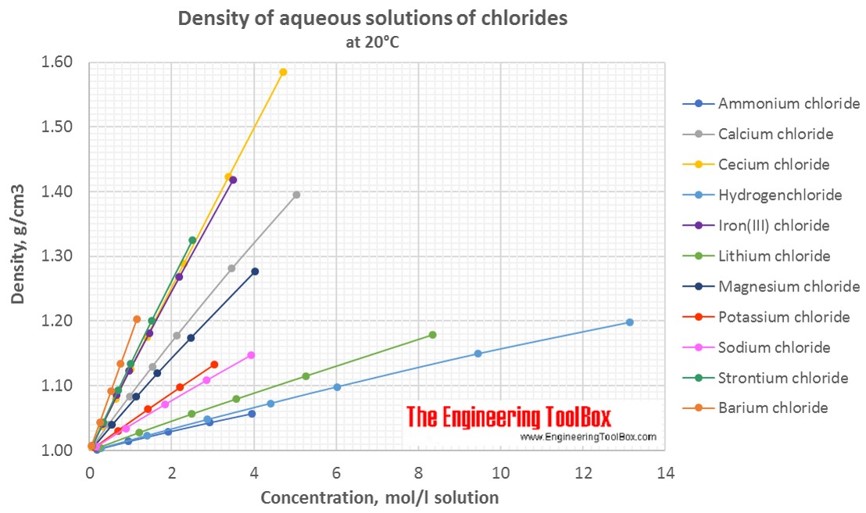
Density of aqueous solutions at 20°C, given as g/cm3:
For full table with Iron, Lithium, Magnesium, Potassium, Sodium and Strontium Chloride - rotate the screen!
| Mass% | Ammonium chloride | Barium chloride | Calcium chloride | Cecium chloride | Hydrogen-chloride | Iron(III) chloride | Lithium chloride | Magnesium chloride | Potassium chloride | Sodium chloride | Strontium chloride |
| 1 | 1.0014 | 1.007 | 1.0065 | 1.0058 | 1.0031 | 1.0068 | 1.0041 | 1.0062 | 1.0046 | 1.0053 | 1.0071 |
| 5 | 1.0138 | 1.0434 | 1.0401 | 1.0374 | 1.0228 | 1.0408 | 1.0272 | 1.0394 | 1.0304 | 1.034 | 1.0437 |
| 10 | 1.0286 | 1.0921 | 1.0835 | 1.0798 | 1.0476 | 1.0853 | 1.056 | 1.0826 | 1.0633 | 1.0707 | 1.0925 |
| 20 | 1.0567 | 1.2031 | 1.1775 | 1.1756 | 1.098 | 1.1816 | 1.115 | 1.1742 | 1.1328 | 1.1478 | 1.2008 |
| 30 | 1.2816 | 1.2885 | 1.1492 | 1.2679 | 1.1791 | 1.2763 | 1.3248 | ||||
| 40 | 1.3957 | 1.4226 | 1.1977 | 1.4176 | |||||||
| 50 | 1.5846 | ||||||||||
| Density at 20°C, given as g/cm3 | |||||||||||
Conversion of the concentration from mass% to mol/kg (moles of solute/kg of water = molality):
For full table with Iron, Lithium, Magnesium, Potassium, Sodium and Strontium Chloride - rotate the screen!
| Mass% | Ammonium chloride | Barium chloride | Calcium chloride | Cecium chloride | Hydrogen-chloride | Iron(III) chloride | Lithium chloride | Magnesium chloride | Potassium chloride | Sodium chloride | Strontium chloride |
| 1 | 0.189 | 0.049 | 0.091 | 0.06 | 0.277 | 0.062 | 0.238 | 0.106 | 0.135 | 0.173 | 0.064 |
| 5 | 0.984 | 0.253 | 0.474 | 0.313 | 1.444 | 0.324 | 1.241 | 0.553 | 0.706 | 0.901 | 0.332 |
| 10 | 2.077 | 0.534 | 1.001 | 0.66 | 3.047 | 0.685 | 2.621 | 1.167 | 1.490 | 1.901 | 0.701 |
| 20 | 4.674 | 1.201 | 2.2553 | 1.485 | 6.857 | 1.54 | 5.897 | 2.626 | 3.353 | 4.278 | 1.577 |
| 30 | 3.862 | 2.546 | 11.754 | 2.398 | 10.109 | 4.501 | 2.703 | ||||
| 40 | 6.007 | 3.96 | 18.284 | 4.11 | |||||||
| 50 | 5.94 | ||||||||||
| Molality at 20°C, given as mol/kgwater | |||||||||||
Conversion of the concentration from mass% to mol/liter (moles of solute/liter of solution = molarity):
For full table with Iron, Lithium, Magnesium, Potassium, Sodium and Strontium Chloride - rotate the screen!
| Mass% | Ammonium chloride | Barium chloride | Calcium chloride | Cecium chloride | Hydrogen-chloride | Iron(III) chloride | Lithium chloride | Magnesium chloride | Potassium chloride | Sodium chloride | Strontium chloride |
| 1 | 0.187 | 0.048 | 0.091 | 0.06 | 0.275 | 0.062 | 0.237 | 0.106 | 0.135 | 0.172 | 0.064 |
| 5 | 0.948 | 0.251 | 0.469 | 0.308 | 1.403 | 0.321 | 1.211 | 0.546 | 0.691 | 0.885 | 0.329 |
| 10 | 1.923 | 0.524 | 0.976 | 0.641 | 2.873 | 0.669 | 2.491 | 1.137 | 1.426 | 1.832 | 0.689 |
| 20 | 3.95 | 1.156 | 2.122 | 1.397 | 6.023 | 1.457 | 5.26 | 2.467 | 3.039 | 3.928 | 1.515 |
| 30 | 3.464 | 2.296 | 9.456 | 2.189 | 8.344 | 4.022 | 2.507 | ||||
| 40 | 5.03 | 3.38 | 13.14 | 3.496 | |||||||
| 50 | 4.706 | ||||||||||
| Molarity at 20°C, given as mol/l solution | |||||||||||
Related Topics
-
Densities
Densities of solids, liquids and gases. Definitions and convertion calculators. -
Material Properties
Properties of gases, fluids and solids. Densities, specific heats, viscosities and more.
Related Documents
-
API Gravity
API expresses the gravity or density of liquid petroleum products. Online API to Specific Gravity calculator. -
Concentration Units Converter
Calculator and formulas for conversion between different units of concentration: Molarity, molality, mole fraction, weight percent of solute and grams of solute per liter of solution - descriptive terms for solubility. -
Crude Oil - Density vs. Temperature
Variations in crude oil density are shown as function of temperatur, together with volume correction factors. -
Densities of Aqueous Solutions of Inorganic Potassium Salts
Changes in density of aqueous solutions with changes in concentration at 20°C. Density of potassium salts in water is plotted as function of wt%, mol/kg water and mol/l solution. -
Densities of Aqueous Solutions of Inorganic Sodium Salts
Changes in density of aqueous solutions with changes in concentration at 20°C. Density of inorganic sodium salts in water is plotted as function of wt%, mol/kg water and mol/l solution. -
Densities of Aqueous Solutions of Organic Acids
Changes in density of aqueous solutions with changes in concentration at 20°C. Density of acetic acid, citric acid, formic acid, D-lactic acid, oxalic acid and trichloroacetic acid in water is plotted as function of wt%, mol/kg water and mol/l solution. -
Density of Aqueous Solutions of Organic Substances as Sugars and Alcohols
Changes in density of aqueous solutions with changes in concentration at 20°C. Density of some sugars, alcohols and other organic substances in water is plotted as function of wt%, mol/kg water and mol/l solution. -
Density of Aqueous Solutions of some Inorganic Substances
Changes in density of aqueous solutions with changes in concentration at 20°C. Density of inorganic substances in water is plotted as function of wt%, mol/kg water and mol/l solution. -
Density vs. Specific Weight and Specific Gravity
An introduction to density, specific weight and specific gravity. -
Elements of the Periodic System
The elements of the periodic system with names, symbols, atomic numbers and weights, melting and boiling points, density, electronegativity and electron affinity, and electron configuration. -
Fuel Oils - Densities vs. Temperature
Variations in fuel oils density as function of temperatur, together with volume correction factors. -
Hydrocarbons, Linear Alcohols and Acids - Densities
Density of hydrocarbons like alcohols and acids as function of carbon number at 20°C / 68°. -
Jet Fuel - Density vs. Temperature
Variations in jet fuel density as function of temperatur, together with volume correction factors. -
Liquid-Liquid Solution - Shrinkage and Estimation of Density
It is possible to estimate the density of a liquid-liquid solution from the density of the solute and the solvent. However, due to shrinkage, the estimate will be a bit too low. -
Liquids - Densities
Densities of common liquids like acetone, beer, oil, water and more. -
Liquids - Densities vs. Pressure and Temperature Change
Densities and specific volume of liquids vs. pressure and temperature change. -
Liquids - Specific Gravities
Specific gravities of liquids like alcohol, oils, benzene, water and many more. -
Lubricating Oil - Densities vs. Temperature
Variations in lubricating oil density as function of temperatur, together with volume correction factors. -
Organic Sulfur Compounds - Densities
Liquid density of different kinds of organic sulfur compounds with varying carbon number (20°C/68°F). Comparison of thiols, sulfides, disulfides and thiophenes. -
Solids - Densities
Densities of selected solids. -
Solutions, Molarity and Dilution
Definitions and examples of how to calculate wt%, molarity and how to prepare dilutions. -
Water - Specific Volume vs. Temperature
Online calculator, figures and tables showing Specific Volume of water at temperatures ranging from 0-370 °C and 32 - 700 °F - Imperial and IS Units.




