Water vs. Steam - Critical and Triple Points
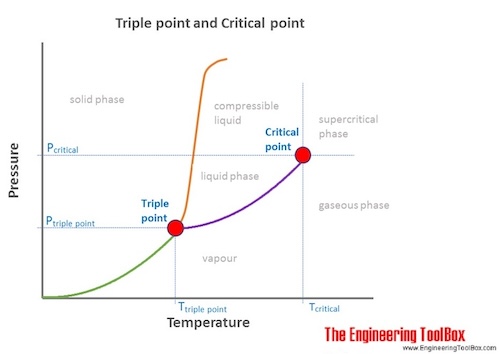
Critical point is where vapor and liquid are indistinguishable and triple point is where ice, water and vapor coexist in thermodynamic equilibrium.
At the critical point there is no change of state when pressure is increased or if heat is added. At the critical point water and steam can't be distinguished and there is no point referring to water or steam.
The critical point of water is achieved at
- Water vapor pressure of 217.75 atm = 220.64 bar = 22.064 MPa = 3200.1 psi
- Temperature of 647.096 K = 373.946 °C = 705.103 °F
- Critical point density: 0.322 g/cm3 = 0.6248 slug/ft3 = 20.102 lbm /ft3
For states above the critical point the steam is supercritical . Supercritical is not the same as superheated - which is saturated steam at lower pressures and temperatures heated above the saturation temperature.
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.
The triple point of water is at
- Water vapor pressure of 0.00604 atm = 6.12 mbar = 611.657 Pa = 0.08871 psi
- Temperature 273.16 K = 0.01 °C = 32.02 °F
- Triple point density (liquid): 0.99979 g/cm3 = 1.93991 slug/ft3 = 62.4148 lbm /ft3
See Water and Heavy Water - for thermodynamic properties.
See also Water Boiling points at high pressure , Boiling points at vacuum pressure , Density, specific weight and thermal expansion coefficient , Dynamic and kinematic viscosity , Enthalpy and entropy , Heat of vaporization , Ionization Constant, pK w , of normal and heavy water , Melting points at high pressure , Saturation pressure , Specific gravity , Specific heat (heat capacity) and Specific volume for online calculatores, figures and tables showing temperature or pressure dependences of the different properties.
Related Topics
-
Fluid Mechanics
The study of fluids - liquids and gases. Involving velocity, pressure, density and temperature as functions of space and time. -
Material Properties
Properties of gases, fluids and solids. Densities, specific heats, viscosities and more. -
Steam and Condensate
Design of steam & condensate systems with properties, capacities, sizing of pipe lines, system configuration and more. -
Thermodynamics
Calculate heat, work, temperature and energy. The thermodynamics of steam and condensate systems. Water and Ice properties. -
Thermodynamics
Work, heat and energy systems. -
Water Systems
Design of hot and cold water service and utility systems with properties, capacities, sizing of pipe lines and more.
Related Documents
-
Critical Points for some Substances
Critical points of some common substances like air, argon, helium and more -
Critical Temperatures and Pressures for some Common Substances
Critical temperatures and pressures for some common substances like air, alcohol, ether, oxygen and more. -
Gases Solved in Water - Diffusion Coefficients
Diffusion flux [kg/m2s] tells how fast a substanse solved in another substance flows due to concentration gradients. Diffusion constants [m2/s] for several gases in water. -
Heavy Water - Thermophysical Properties
Thermodynamic properties of heavy water (D2O) like density, melting temperature, boiling temperature, latent heat of fusion, latent heat of evaporation, critical temperature and more. -
Superheated Steam - Enthalpy
Enthalpy of steam superheated to temperatures above it's boiling point. -
Superheated Steam - Entropy
The entropy of steam superheated to temperatures above saturation points. -
Triple Points Substances
Triple points for common substances. -
Vacuum Pipes - Pressure Loss vs. Air Flow
Calculate pressure drops in vacuum pipe lines. -
Water - Boiling Points at Higher Pressures
Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). Temperature given as °C, °F, K and °R. -
Water - Boiling Points at Vacuum Pressure
Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, SI and Imperial units. -
Water - Density, Specific Weight and Thermal Expansion Coefficients
Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360°C (32 to 680°F). -
Water - Dynamic (Absolute) and Kinematic Viscosity vs. Temperature and Pressure
Free online calculator - figures and tables with viscosity of water at temperatures ranging 0 to 360°C (32 to 675°F) - Imperial and SI Units. -
Water - Enthalpy and Entropy vs. Temperature
Figures and tables showing the enthalpy and entropy of liquid water as function of temperature - SI and Imperial Units. -
Water - Ionization Constant, pKw, of Normal and Heavy Water
Ionization constant (= dissociation constant = self-ionization constant = ion product = autoprotolysis constant ) of water and heavy water, given as function of temperature (°C and °F) in figures and tables. -
Water - Properties at Gas-Liquid Equilibrium Conditions
Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, Prandtl number, thermal diffusivity, entropy and enthalpy). -
Water - Saturation Pressure vs. Temperature
Online calculator, figures and tables with water saturation (vapor) pressure at temperatures ranging 0 to 370 °C (32 to 700°F) - in Imperial and SI Units. -
Water - Specific Gravity vs. Temperature
Figures and tables showing specific gravity of liquid water in the range of 32 to 700 °F or 0 to 370°C, using water density at four different temperatures as reference. -
Water - Specific Heat vs. Temperature
Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 °C (32-700 °F) - SI and Imperial units. -
Water - Specific Volume vs. Temperature
Online calculator, figures and tables showing Specific Volume of water at temperatures ranging from 0-370 °C and 32 - 700 °F - Imperial and IS Units. -
Water - Thermal Conductivity vs. Temperature
Figures and tables showing thermal conductivity of water (liquid and gas phase) with varying temperature and pressure, SI and Imperial units. -
Water - Thermophysical Properties
Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting, latent heat of evaporation, critical temperature and more. -
Water-Steam Mollier Diagram
Enthalpy-entropy diagram for water and steam. -
Wet Steam - Enthalpy
Wet steam, dryness fraction and enthalpy. -
Wet Steam - Quality vs. Dryness Fractions
Introduction and definition of steam quality and dryness fraction including calculating wet steam enthalpy and specific volume.




