Carnot Efficiency
The efficiency of the Carnot cycle.
An ideal reversible cycle where heat is taken in at constant upper temperature and rejected at constant lower temperature was suggested by Sadi Carnot. The theoretically most efficient heat engine cycle, the Carnot cycle, consists of
- two isothermal processes and
- two adiabatic processes
Since the second law of thermodynamics states that not all supplied heat in a heat engine can be used to do work the Carnot efficiency limits the fraction of heat that can be used.
The Carnot efficiency can be expressed as
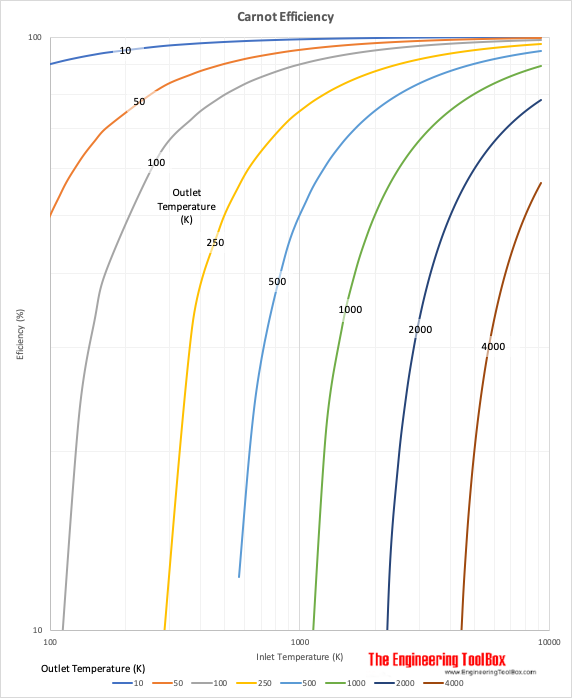
μC = (Ti - To) / Ti (1)
where
μC = efficiency of the Carnot cycle
Ti = temperature at the engine inlet (K)
To = temperature at engine exhaust (K)
The wider the range of temperature, the more efficient becomes the cycle. The lowest temperature is limited by the temperature of the sink of heat - if it is the atmosphere or the ocean, river or whatever available. Normally the lowest temperature available is in the range 10 - 20 oC. The maximum temperature is limited by the metallurgical strength of the available materials.
Related Topics
-
Thermodynamics
Work, heat and energy systems.
Related Documents
-
1st Law of Thermodynamics
The First Law of Thermodynamics simply states that energy can be neither created nor destroyed (conservation of energy). Thus power generation processes and energy sources actually involve conversion of energy from one form to another, rather than creation of energy from nothing. -
2nd Law of Thermodynamics
Entropy and disorder. -
Condensation of Steam - Heat Transfer
Heat transfer when steam condensates. -
Dalton's Law
Gibbs' Dalton's law of the total pressure of a mixture of gases. -
Efficiency
The measure of usefulness. -
Efficiency
Efficiency is the ratio useful energy output to energy input. -
Energy Transfer Equation
Fluid energy transfer. -
Heat, Work and Energy
Heat vs. work vs. energy. -
Heating Up Applications - Energy Required and Heat Transfer Rates
Energy required to heat up a substance. -
Power Plants - Performance Efficiencies
Power plants heat rates, thermal efficiencies, capacity factors, load factors, economic efficiencies, operational efficiencies and energy efficiencies. -
Rankine Efficiency
The efficiency of the Rankine cycle. -
Thermal Resistivity and Conductivity
The ability of a material to resist flow of heat. -
Thermal Transmittance vs. Thermal Resistance
The thermal transmittance U vs. the thermal resistance R.




