Organic Sulfur Compounds - Physical Data
Boiling and melting points of thoils, sulfides, disulfides and thiophenes shown together with molecular structures, as well as molweights and density.
The figures below show the boiling and melting point for organic sulfur compounds as sulfides, disulfides, thiols (mercaptans) and thiophenes, together with the molecular structures of the different compounds. The table shows the same numbers together with the molecular weight and density, as well as the numbers of carbon, hydrogen and sulfur in the molecules. All numbers are given for boiling and melting at 1 atm (760 mm Hg). For some compounds the boiling point at 1 atm is not available. For these, the 1 atm boiling point is estimated from low pressure measurements, using a vaporization heat of 54- 70 kJ/mol (increasing with increasing BP). It is not known whether these compounds are stable at the 1 atm boiling temperature or not. Definitions of the organic classes are given below the figures.
- Boiling point - the temperature at which a liquid turns into a gas
- Melting point - the temperature at which a solid turns into a liquid
See also similar figures and tables for organic nitrogen compounds and boiling and melting point of hydrocarbons, alcohols and acids, as well as densities of organic sulfur compounds.
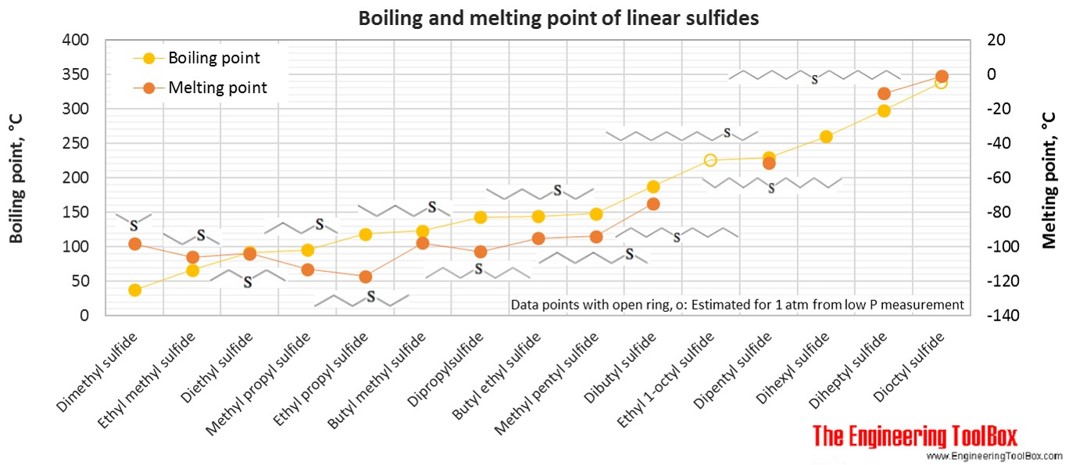
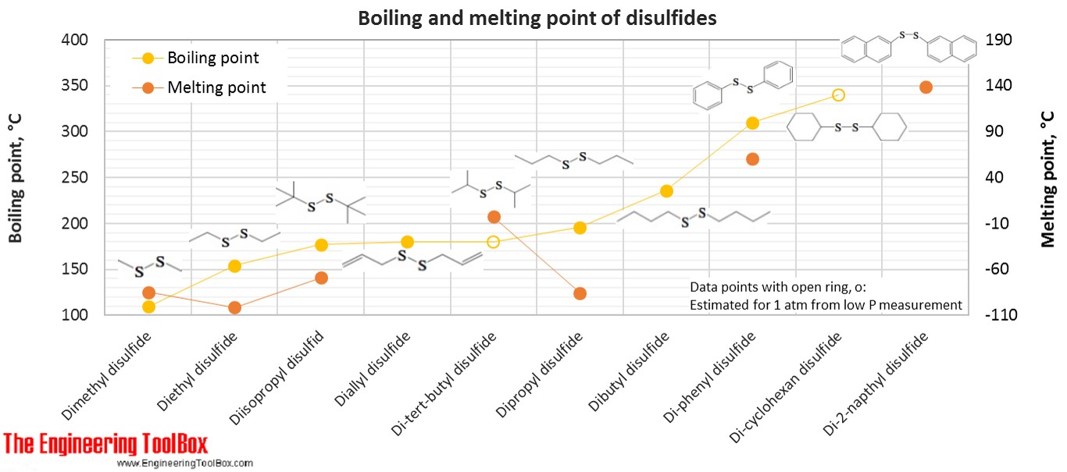

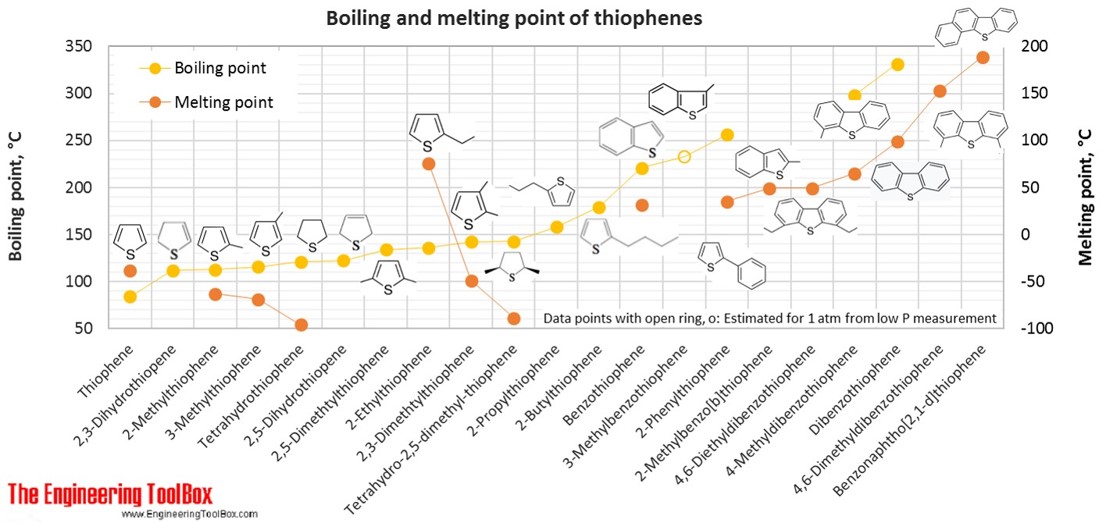
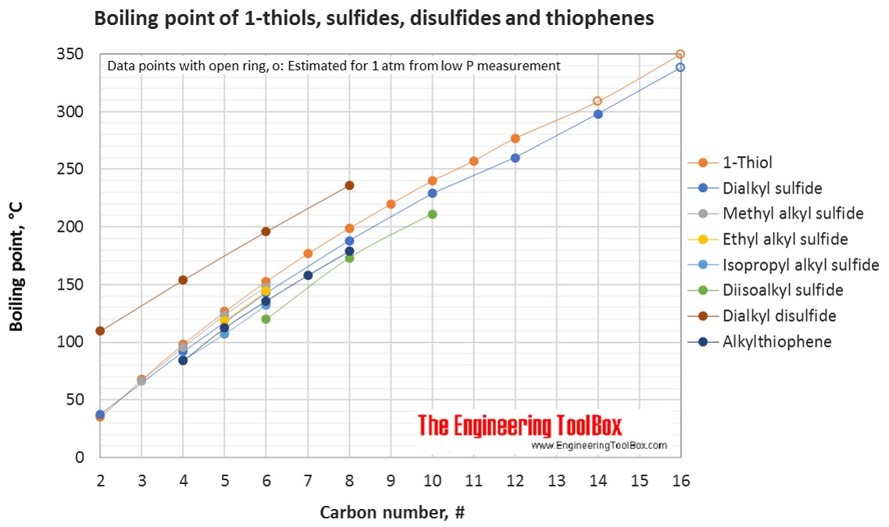
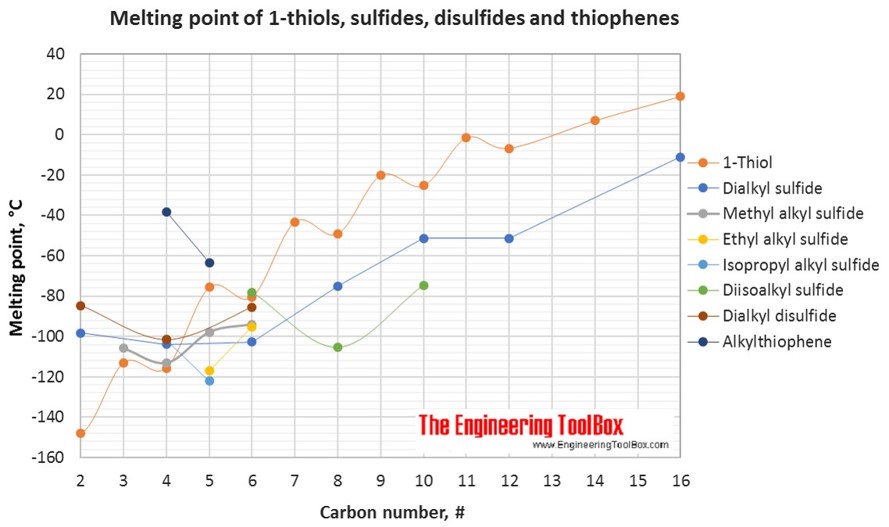
For full table with molweight and boling point - rotate the screen!
| Group | Compound name | Common name | #C | #H | #S | Molweight [g/mol] | Melting point [°C] | Boiling point [°C] | Density @20°C [g/ml] |
|---|---|---|---|---|---|---|---|---|---|
| 1-Thiol | Methanethiol | Methyl mercaptan | 1 | 4 | 1 | 48.11 | -123 | 6 | 0.866 |
| 1-Thiol | Ethanethiol | Ethyl mercaptan | 2 | 6 | 1 | 62.13 | -148 | 35 | 0.835 |
| 1-Thiol | 1-Propanethiol | Propyl mercaptan | 3 | 8 | 1 | 76.16 | -113 | 68 | 0.841 |
| 1-Thiol | 1-Butanethiol | Butyl mercaptan | 4 | 10 | 1 | 90.19 | -116 | 98 | 0.842 |
| 1-Thiol | 1-Pentanethiol | Pentyl mercaptan | 5 | 12 | 1 | 104.21 | -76 | 127 | 0.844 |
| 1-Thiol | 1-Hexanethiol | Hexyl mercaptan | 6 | 14 | 1 | 118.24 | -81 | 153 | 0.842 |
| 1-Thiol | 1-Heptanethiol | Heptyl mercaptan | 7 | 16 | 1 | 132.27 | -43 | 177 | 0.843 |
| 1-Thiol | 1-Octanethiol | Octyl mercaptan | 8 | 18 | 1 | 146.29 | -49 | 199 | 0.843 |
| 1-Thiol | 1-Nonanethiol | Nonyl mercaptan | 9 | 20 | 1 | 160.32 | -20 | 220 | 0.846 |
| 1-Thiol | 1-Decanethiol | Decyl mercaptan | 10 | 22 | 1 | 174.35 | -25 | 240 | 0.844 |
| 1-Thiol | 1-Undecanethiol | Undecyl mercaptan | 11 | 24 | 1 | 188.37 | -2 | 257 | 0.845 |
| 1-Thiol | 1-Dodecanethiol | Dodecyl mercaptan | 12 | 26 | 1 | 202.40 | -7 | 277 | 0.844 |
| 1-Thiol | 1-Tetradecanethiol | Tetradecyl mercaptan | 14 | 30 | 1 | 230.45 | 7 | 309* | 0.850 |
| 1-Thiol | 1-Hexadecanethiol | Cetyl mercaptan | 16 | 34 | 1 | 258.51 | 19 | 350* | |
| Branched thiol | 2-Propanethiol | Isopropyl mercaptan | 3 | 8 | 1 | 76.16 | -131 | 53 | 0.814 |
| Branched thiol | 2-Butanethiol | sec-Butyl mercaptan | 4 | 10 | 1 | 90.19 | -165 | 85 | 0.830 |
| Branched thiol | 2-Pentanethiol | sec-Pentyl mercaptan | 5 | 12 | 1 | 104.21 | -169 | 113 | 0.833 |
| Branched thiol | 3-Pentanethiol | 3-Pentyl mercaptan | 5 | 12 | 1 | 104.21 | -111 | 116 | 0.841 |
| Branched thiol | Benzenethiol | Phenyl mercaptan | 6 | 6 | 1 | 110.18 | -15 | 169 | 1.078 |
| Branched thiol | 2-Hexanethiol | 7 | 8 | 1 | 118.24 | -147 | 139 | 0.835 | |
| Linear sulfide | Dimethyl sulfide | 2-Thiapropane | 2 | 6 | 1 | 62.13 | -98 | 37 | 0.848 |
| Linear sulfide | Ethyl methyl sulfide | 3 | 8 | 1 | 76.16 | -106 | 67 | 0.842 | |
| Linear sulfide | Diethyl sulfide | Ethyl sulfide | 4 | 10 | 1 | 90.19 | -104 | 92 | 0.836 |
| Linear sulfide | Methyl propyl sulfide | 4 | 10 | 1 | 90.19 | -113 | 96 | 0.842 | |
| Linear sulfide | Ethyl propyl sulfide | 5 | 12 | 1 | 104.21 | -117 | 119 | 0.837 | |
| Linear sulfide | Butyl methyl sulfide | 5 | 12 | 1 | 104.21 | -98 | 123 | 0.843 | |
| Linear sulfide | Dipropylsulfide | 6 | 14 | 1 | 118.24 | -103 | 143 | 0.838 | |
| Linear sulfide | Butyl ethyl sulfide | 6 | 14 | 1 | 118.24 | -95 | 144 | 0.838 | |
| Linear sulfide | Methyl pentyl sulfide | 6 | 14 | 1 | 118.24 | -94 | 148 | 0.843 | |
| Linear sulfide | Dibutyl sulfide | Butyl sulfide | 8 | 18 | 1 | 146.29 | -75 | 188 | 0.839 |
| Linear sulfide | Ethyl 1-octyl sulfide | 1-(Ethylthio)octane | 10 | 22 | 1 | 174.35 | 225* | ||
| Linear sulfide | Dipentyl sulfide | 10 | 22 | 1 | 174.35 | -51 | 229 | 0.841 | |
| Linear sulfide | Dihexyl sulfide | Hexyl sulfide | 12 | 26 | 1 | 202.40 | 260 | 0.841 | |
| Linear sulfide | Diheptyl sulfide | Heptyl sulfide | 14 | 30 | 1 | 230.45 | -11 | 298 | 0.842 |
| Linear sulfide | Dioctyl sulfide | Octyl sulfide | 16 | 34 | 1 | 258.51 | -1 | 338* | 0.844 |
| Branched sulfide | Isopropyl methyl sulfide | 4 | 10 | 1 | 90.19 | -101 | 85 | 0.829 | |
| Branched sulfide | Tert-butyl methyl sulfide | 5 | 12 | 1 | 104.21 | 99 | 0.830 | ||
| Branched sulfide | Ethyl isopropyl sulfide | 5 | 12 | 1 | 104.21 | -122 | 107 | 0.825 | |
| Branched sulfide | Diallyl sulfide | 6 | 10 | 1 | 114.21 | -85 | 138 | 0.893 | |
| Branched sulfide | Diisopropyl sulfid | 6 | 14 | 1 | 118.24 | -78 | 120 | 0.814 | |
| Branched sulfide | Tert-butyl ethyl sulfide | 6 | 14 | 1 | 118.24 | -86 | 120 | 0.820 | |
| Branched sulfide | Isopropyl propyl sulfid | 6 | 14 | 1 | 118.24 | 132 | 0.827 | ||
| Branched sulfide | Methyl tert-pentyl sulfide | 6 | 14 | 1 | 118.24 | 150 | 0.840 | ||
| Branched sulfide | Methyl phenyl sulfide | Methyl thiobenzene, Thianisole | 7 | 8 | 1 | 124.20 | -15 | 188 | 1.057 |
| Branched sulfide | Phenyl vinyl sulfide | (Phenylthio)ethylene | 8 | 8 | 1 | 136.21 | 180 | 1.044 | |
| Branched sulfide | Ethyl phenyl sulfide | Ethyl thiobenzene | 8 | 10 | 1 | 138.23 | 204 | 1.023 | |
| Branched sulfide | Di-tert-butyl sulfide | 8 | 18 | 1 | 146.29 | -9 | 152 | 0.819 | |
| Branched sulfide | Di-sec-butyl sulfide | 8 | 18 | 1 | 146.29 | 167 | 0.835 | ||
| Branched sulfide | Diisobutyl sulfid | 8 | 18 | 1 | 146.29 | -106 | 173 | 0.829 | |
| Branched sulfide | Allyl phenyl sulfide | 9 | 10 | 1 | 150.24 | 224 | 1.024 | ||
| Branched sulfide | Diisopentyl sulfid | 10 | 22 | 1 | 174.35 | -75 | 211 | 0.832 | |
| Branched sulfide | Diphenyl sulfide | Phenyl sulfide | 12 | 10 | 1 | 186.27 | -15 | 294 | 1.114 |
| Branched sulfide | Dibenzyl sulfide | Benzyl sulfide | 14 | 14 | 1 | 214.33 | 48 | 335 | 1.058 |
| Disulfid | Dimethyl disulfide | DMDS | 2 | 6 | 2 | 94.20 | -85 | 110 | 1.063 |
| Disulfid | Diethyl disulfide | 4 | 10 | 2 | 122.25 | -102 | 154 | 0.993 | |
| Disulfid | Diallyl disulfide | DADS Garlicin | 6 | 10 | 2 | 146.27 | 180 | 1.010 | |
| Disulfid | Diisopropyl disulfid | 6 | 14 | 2 | 150.31 | -69 | 177 | 0.944 | |
| Disulfid | Dipentyl disulfide | 10 | 22 | 2 | 206.41 | ||||
| Disulfid | Dihexyl disulfide | 12 | 26 | 2 | 234.46 | ||||
| Disulfid | Diheptyl disulfide | 14 | 30 | 2 | 262.52 | ||||
| Disulfid | Dioctyl disulfide | 16 | 34 | 2 | 290.57 | ||||
| Disulfid | Dipropyl disulfide | 6 | 14 | 2 | 150.31 | -85 | 196 | 0.960 | |
| Disulfid | Di-tert-butyl disulfide | 8 | 18 | 2 | 178.36 | -3 | 180* | 0.923 | |
| Disulfid | Dibutyl disulfide | 8 | 18 | 2 | 178.36 | 236 | 0.938 | ||
| Disulfid | Di-phenyl disulfide | 12 | 10 | 2 | 218.34 | 60 | 310 | 1.353 | |
| Disulfid | Di-cyclohexan disulfide | 12 | 22 | 2 | 230.43 | 340* | 1.049 | ||
| Disulfid | Di-2-napthyl disulfide | 20 | 14 | 2 | 318.46 | 139 | 1.144 | ||
| Thiophene | Thiophene | Thiofurane | 4 | 4 | 1 | 84.14 | -38 | 84 | 1.065 |
| Thiophene | 2,3-Dihydrothiopene | 4 | 6 | 1 | 86.16 | 112 | |||
| Thiophene | 2,5-Dihydrothiopene | 4 | 6 | 1 | 86.16 | 122 | |||
| Thiophene | Tetrahydrothiophene | Thiolane, Thiophane, Thiacyclopentane | 4 | 8 | 1 | 88.17 | -96 | 121 | 0.999 |
| Thiophene | 2-Methylthiophene | 5 | 6 | 1 | 98.17 | -63 | 113 | 1.019 | |
| Thiophene | 3-Methylthiophene | 5 | 6 | 1 | 98.17 | -69 | 115 | 1.022 | |
| Thiophene | 2,5-Dimethtylthiophene | 6 | 8 | 1 | 112.19 | 134 | 0.987 | ||
| Thiophene | 2-Ethylthiophene | 6 | 8 | 1 | 112.19 | 76 | 136 | 0.993 | |
| Thiophene | 2,3-Dimethtylthiophene | 6 | 8 | 1 | 112.19 | -49 | 142 | 1.007 | |
| Thiophene | Tetrahydro-2,5-dimethyl-thiophene | 6 | 12 | 1 | 116.22 | -89 | 143 | 0.922 | |
| Thiophene | 2-Propylthiophene | 7 | 10 | 1 | 126.22 | 158 | 0.984 | ||
| Thiophene | Benzothiophene | Thianaphthene | 8 | 6 | 1 | 134.20 | 31 | 221 | 1.156 |
| Thiophene | 3-Methylbenzothiophene | 8 | 6 | 1 | 134.20 | 233* | 1.108 | ||
| Thiophene | 2-Butylthiophene | 8 | 12 | 1 | 140.25 | 179 | 0.954 | ||
| Thiophene | 2-Methylbenzo[b]thiophene | 2-Methylthianaphthene | 9 | 8 | 1 | 148.22 | 49 | ||
| Thiophene | 2-Phenylthiophene | 10 | 8 | 1 | 160.24 | 35 | 256 | ||
| Thiophene | Dibenzothiophene | 12 | 8 | 1 | 184.26 | 99 | 332 | 1.252 | |
| Thiophene | 4-Methyldibenzothiophene | 13 | 10 | 1 | 198.28 | 65 | 298 | ||
| Thiophene | 4,6-Dimethyldibenzothiophene | 14 | 12 | 1 | 212.31 | 153 | |||
| Thiophene | Benzonaphtho[2,1-d]thiophene | 16 | 10 | 1 | 234.32 | 189 | |||
| Thiophene | 4,6-Diethyldibenzothiophene | 16 | 16 | 1 | 240.36 | 49 | |||
| * Values estimated for 1 atm from low pressure measurements | |||||||||
Definition of organic compounds
Hydrocarbon: An organic compound consisting entirely of hydrogen and carbon.
Alkane: An acyclic saturated hydrocarbon, with the general formula CnH2n+2. Also called paraffin.
Alkyl: An alkyl group is an alkane substituent missing one hydrogen, with general formula CnH2n+1
Phenyl: An phenyl group is a benzene substituent missing one hydrogen, with general formula C6H5.
Organic sulfur compounds:
Thiol: An organosulfur compound that contains a carbon-bonded sulfhydryl group (-SH), with the general formula R–SH (where R represents an alkyl or other organic substituent). For thiols in this document the R represents an alkyl group, and in a 1-thiol the -SH group is located at the end of the alkyl chain. In a 2-thiol and a 3-thiol the -SH group is located at the second and third carbon atom in the alkyl chain, respectively. Also called mercaptan.
Sulfide: An organic compound of the form R’–S–R’’ (where R’ and R’’ represents an alkyl or other organic substituent).
Dialkyl sulfide: A sulphide where R’ and R’’ are linear alkyl groups, and of the same length.
Methyl alkyl sulfide: A sulphide where R’ is a methyl group and R’’ are any alkyl group.
Ethyl alkyl sulfide: A sulphide where R’ is an ethyl group and R’’ are any alkyl group.
Isopropyl alkyl sulphide: A sulphide where R’ is an isopropyl group and R’’ are any alkyl group.
Diisoalkyl sulphide: A sulphide where R’ and R’’ are isoalkyl groups, and of the same length.
Disulfide: An organic compound of the form R’–S-S–R’’ (where R’ and R’’ represents an alkyl or other organic substituent).
Thiophene: A heterocyclic compound with the formula C4H4S. Consisting of a stable, flat five-membered ring.
Alkylthiophene: A monosubstituted thiophene with one branching via the attachment of one alkyl group on one carbon of the thiophene ring, with the general formula CnH(2n+1)C4H3S
Benzothiophene: A polycyclic aromatic compound consisting of a benzene ring connected to a thiophene ring, with the formula C8H6S.
Related Topics
-
Boiling Points of Fluids
Boiling points of elements, products and chemical species at varying conditions. -
Material Properties
Properties of gases, fluids and solids. Densities, specific heats, viscosities and more. -
Melting and Freezing Points
Melting and freezing points of elements and chemical species at varying conditions.
Related Documents
-
Alcohols and Carboxylic Acids - Physical Data
Molweight, melting and boiling point, density, pKa-values, as well as number of carbon and hydrogen atoms in molecules are given for 150 different alcohols and acids. -
Crude Oil - Density vs. Temperature
Variations in crude oil density are shown as function of temperatur, together with volume correction factors. -
Fuel Oils - Densities vs. Temperature
Variations in fuel oils density as function of temperatur, together with volume correction factors. -
Hydrocarbon Mixtures - Average Boiling Points vs. Gravity and Molecular Weights
Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight. -
Hydrocarbon Mixtures - Molecular Weight vs. Gravity and Average Boiling Point
Formulas and examples of calculation of average molecular weight of hydrocarbon mixtures from gravity and average boiling point, achieved from distillation data. -
Hydrocarbons - Physical Data
Molweight, melting and boiling point, density, flash point and autoignition temperature, as well as number of carbon and hydrogen atoms in each molecule for 200 different hydrocarbons. -
Hydrocarbons - Melting Point vs. Molecular Weight
Calculate melting point of hydrocarbons from molecular weight (molar mass). -
Hydrocarbons, Alcohols and Acids - Boiling points
Boiling temperatures (°C and °F) with varying carbon numbers up to C33. -
Inorganic Compounds in Water - Melting and Boiling Temperature, Density and Solubility
Physical constants for more than 280 common inorganic compounds. Density is given for the actual state at 25°C and for liquid phase at melting point temperature. -
Jet Fuel - Density vs. Temperature
Variations in jet fuel density as function of temperatur, together with volume correction factors. -
Liquid-Liquid Solution - Shrinkage and Estimation of Density
It is possible to estimate the density of a liquid-liquid solution from the density of the solute and the solvent. However, due to shrinkage, the estimate will be a bit too low. -
Liquids - Freezing and Melting Points
Common fluids and their freezing and melting points. -
Lubricating Oil - Densities vs. Temperature
Variations in lubricating oil density as function of temperatur, together with volume correction factors. -
Melting points of Hydrocarbons, Alcohols and Acids
Melting temperature (°C and °F) with carbon number up to C33. -
Naming of Organic Compounds
Nomenclature rules for different groups of organic compounds and functional groups, together with examples of use of the rules. -
Organic Nitrogen Compounds - Physical Data
Boiling and melting points of amines, diamines, pyrroles, pyridines, piperidines and quinolines shown together with their molecular structures, as well as molweights and density. -
Organic Sulfur Compounds - Densities
Liquid density of different kinds of organic sulfur compounds with varying carbon number (20°C/68°F). Comparison of thiols, sulfides, disulfides and thiophenes. -
Petroleum Products - Average Boiling Points
Definition, explanation and examples of calculation of various types of average boiling point of petroleum products and other mixtures of hydrocarbons: VABP, MABP, WABP, CABP and MeABP. -
Yield Structure of Crude Oils with Increasing Density of Crude
Yields of different crude oil distillation cuts are plotted as function of whole crude specific gravity. Fractions based on European and North American markets, and the typical differences in crude oil fractionation in the two markets are also shown.




